| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 15, Number 10-11, December 2023, pages 461-468
Treatment With Antitumor Agents Recommended by Cancer Genome Panel for Uterine Leiomyosarcoma
Takuma Hayashia, b, d, Naoya Kishimotoc, Kaoru Abikoc, Ikuo Konishib, c
aCancer Medicine, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan
bFirst-Track Medical R&D, The Japan Agency for Medical Research and Development (AMED), Tokyo 100-0004, Japan
cObstetrics and Gynecology, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan
dCorresponding Author: Takuma Hayashi, Cancer Medicine, National Hospital Organization Kyoto Medical Center, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan
Manuscript submitted October 18, 2023, accepted December 6, 2023, published online December 20, 2023
Short title: Cancer Genome Medicine for Advanced Leiomyosarcoma
doi: https://doi.org/10.14740/jocmr5052
| Abstract | ▴Top |
To date, cancer genomic medicine, using cancer gene panel covered by health insurance from June 2019, has been performed for advanced malignant tumors under public medical insurance. In gynecology, the first-line treatment for uterine leiomyosarcomas, which is a mesenchymal uterine tumor, is surgery. In uterine leiomyosarcoma cases, recurrence is observed within 2 years postoperatively; however, to date, clinical trials have not shown efficacy with existing antitumor agents. We noted efficacy in two cases with advanced/recurrent uterine leiomyosarcoma using an antitumor agent selected on the basis of cancer gene panel testing results. Following uterine leiomyosarcoma diagnosis, they underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy as standard surgical treatment. After the surgical treatment, the imaging test revealed recurrent tumors; subsequently, they were treated with doxorubicin alone or doxorubicin combined with Gemzar. However, cancer genome gene panel test was performed because the malignant tumor worsened. Based on the cancer genome gene panel test results, the two cases with advanced uterine leiomyosarcoma were associated with increased tumor mutational burden (TMB) or pathogenic variants (PVs) of AKT serine/threonine kinase 1 (AKT1). Therefore, treatment with pembrolizumab, which is a drug covered by insurance for patients with TMB-high, or treatment with kinase inhibitors for patients with PVs in AKT, was considered. Cancer genomic medicine using cancer gene panel provides a new treatment strategy for intractable malignant tumors. This study aimed to discuss the usefulness of cancer genomic medicine by cancer gene panel testing using the cases of advanced and recurrence uterine leiomyosarcoma and the latest findings.
Keywords: Cancer gene testing; Leiomyosarcoma; TMB; ICI; Kinase inhibitor
| Introduction | ▴Top |
Most of the tumors detected in the smooth muscle tissues of the uterine corpus, ovaries, and fallopian tubes are uterine leiomyomas, which are benign mesenchymal tumors [1]. The prevalence of uterine leiomyoma is approximately 70% and more than 80% in white women and women of African ancestry during their lifetime, respectively [2]. Regular screening with annual imaging tests is performed for several uterine leiomyoma cases [3]. Uterine sarcomas are malignant tumors arising from the smooth muscle tissue or connective tissue of the uterus. Endometrial stromal sarcomas are malignant tumors that develop from the stromal tissue of the uterine lining, and their cells contain more epithelial tissue components than smooth muscle tissue components [4]. Uterine leiomyosarcomas are malignant uterine tumors that develop from the uterine smooth muscle layer; the 5-year survival rate for uterine leiomyosarcomas is < 20% [5, 6]. Uterine carcinosarcomas are malignant tumors wherein a lesion containing malignant tumor cells originate from the epithelial cells of the endometrial lining of the uterine corpus [7]. Uterine carcinosarcomas were also previously called malignant mixed mesodermal/Mullerian tumor.
In actual clinical practice, the cells of uterine mesenchymal tumors have a diverse cell and nuclear morphology; therefore, differential diagnosis between uterine leiomyosarcomas, which are malignant tumors, and other uterine mesenchymal tumors, including uterine leiomyomas, is difficult [8]. In several cases, uterine leiomyosarcomas coexist with uterine leiomyomas. Furthermore, owing to the high prevalence of uterine leiomyomas, the diagnosis of uterine leiomyosarcoma, particularly before surgery, is extremely challenging [8, 9]. To date, clinical trials conducted by various medical teams have investigated the effectiveness of antitumor agents on uterine smooth muscle tumors; however, the effectiveness of various antitumor agents against uterine leiomyosarcomas is limited [10, 11]. Unfortunately, no common treatment has been established for uterine leiomyosarcomas. Therefore, currently, cases wherein pathogenic variants (PVs) (also called draggable variants) of uterine leiomyosarcomas are detected using cancer gene panel testing, such as FoundationOne® CDx tissue or liquid (FoundationOne® CDx’s cancer genome test, Foundation Medicine, Inc., Cambridge, MA, USA), are treated by prescribing PV-targeting antitumor drugs [12, 13].
To explore PV-targeting antitumor drugs, our medical team performed FoundationOne® CDx tissue cancer gene panel test on two cases with advanced/recurrent uterine leiomyosarcoma that worsened during chemotherapy following surgical treatment. In our medical institution, the FoundationOne® CDx tissue cancer gene panel test detected PVs in the serine-threonine kinase AKT molecule in the cells of an advanced/recurrent uterine leiomyosarcoma [14]. Conversely, tumor mutational burden (TMB)-high (Supplementary Material 1, www.jocmr.org) was detected in the cells of an advanced/recurrent uterine leiomyosarcoma in another patient [15, 16]. Therefore, pazopanib or pembrolizumab, a multikinase inhibitor, was administered to each patient with advanced/recurrent uterine leiomyosarcoma. Currently, each patient with advanced/recurrent uterine leiomyosarcoma is treated with an antitumor drug selected on the basis of the cancer gene panel test results. Surgery is the first-line treatment for uterine leiomyosarcomas. However, the follow-up treatment following surgical treatment, treatment with existing antitumor drugs, or radiation therapy for progressive/recurrent uterine leiomyosarcoma has not been established. Under this clinical condition, we confirmed that an antitumor agent selected on the basis of the cancer genome gene panel test results was effective against advanced/recurrent uterine leiomyosarcoma. Therefore, we report two cases of uterine leiomyosarcoma who responded well to antitumor agents selected on the basis of the cancer genome gene panel test results.
| Case Report | ▴Top |
Ethics approval and consent to participate
The two patients with advanced/recurrent uterine leiomyosarcoma reported in this Case Report are participating in two ethical committee-approved clinical studies. The medical ethics education course completion numbers for each author are AP0000151756, AP0000151757, AP0000151769, and AP000351128. As this research was considered clinical research, consent to participate was required. After briefing regarding the clinical study and approval of the research contents, the participants signed an informed consent form.
Clinical research
This is a multi-center retrospective observational clinical study of subjects who underwent cancer genomic medicine at a cancer medical facility in Kyoto, Japan. This study was reviewed and approved by the Central Ethics Review Board of the National Hospital Organization Headquarters in Japan (Tokyo, Japan) on November 18, 2020, and Kyoto University School of Medicine (Kyoto, Japan) on August 24, 2022, with approval codes NHO R4-04 and M237.
This is a study to establish method of prognostic prediction for uterine mesenchymal tumor by immunohistological biomarkers at multicenter joint research. This study was reviewed and approved by the Central Ethics Review Board of the National Hospital Organization Headquarters in Japan (Tokyo, Japan) on November 8, 2019, and Kyoto University School of Medicine (Kyoto, Japan) on August 25, 2023, with approval codes NHO H31-02 and M192. All participants agreed to take part in the present study.
Magnetic resonance imaging
To determine the presence, size, and location of the patient’s mass, contrast-enhanced (magnetic resonance imaging) MRI was performed using contrast-enhanced MRI equipment (Vantage Centurian: Vantage Galan 3T MRT-3020, Canon Medical Systems, Inc., Ohtawara, Tochigi, Japan).
Cancer genomic testing
Following surgical treatment, the imaging test revealed recurrent tumors, which were treated with doxorubicin (DXR) alone or DXR combined with Gemzar. However, to explore PV-targeting antitumor drugs, our medical team performed FoundationOne® CDx tissue cancer gene panel test (Foundation Medicine, Inc., Cambridge, MA, USA) using tissue sections of the resected advanced/recurrent uterine leiomyosarcoma that worsened during chemotherapy following surgical treatment.
Case 1
This was a 55-year-old female with a performance status (PS) scale (Supplementary Material 2, www.jocmr.org) of 0, diagnosed with uterine leiomyosarcoma recurrence and right hydronephrosis, who had no significant medical history, but reported a family history of stomach cancer in her grandmother.
In 2014, uterine leiomyoma development was noted. She subsequently received regular follow-up at the nearby obstetrics and gynecology clinic; however, no changes were observed until June 14, 2021. However, uterine malignancy was suspected on computed tomography (CT). Therefore, on July 12, 2021, she was referred to the Department of Gynecological Tumor at National Hospital Organization Kyoto Medical Center (Kyoto, Kyoto, Japan). She was diagnosed with uterine sarcoma stage I on contrast-enhanced MRI and positron emission tomography (PET)-CT.
On July 29, 2021, she underwent total abdominal hysterectomy, bilateral adnexectomy, partial omentectomy, and small mesenteric disseminated lesion resection.
She was subsequently diagnosed with uterine leiomyosarcoma stage Ib on pathological examination (Fig. 1). The initial treatment was completed using the surgical treatment recommended by clinical guidelines, including the National Comprehensive Cancer Network®. Following hospital discharge, she was followed up on an outpatient visit.
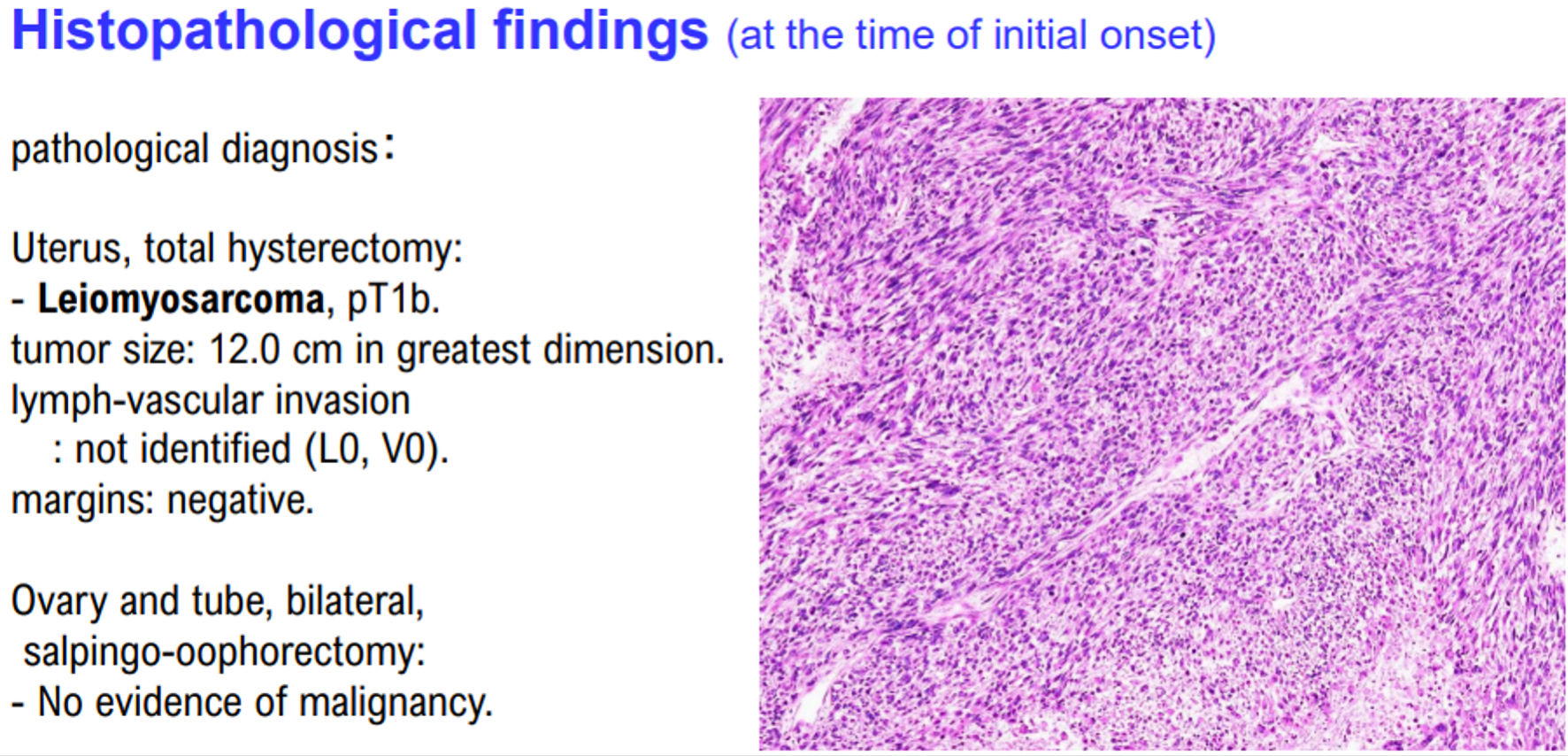 Click for large image | Figure 1. Histopathological findings of case 1. The patient in case 1 was diagnosed with uterine leiomyosarcoma by histopathological examination. Histopathological examination revealed no malignant tumor cells in the ovaries and fallopian tubes. |
On June 27, 2022, contrast-enhanced CT revealed intraperitoneal disseminated tumor and metastases to the lungs, and tumor recurrence was diagnosed (intraperitoneal disseminated tumor, tumor size: 2.1 cm; pelvic tumor size: 1.0 cm; multiple lung metastases, including 7-mm tumor in the left lung and 4-mm tumor in the right lung. Contrast-enhanced CT revealed other microlesions) (Figs. 2, 3).
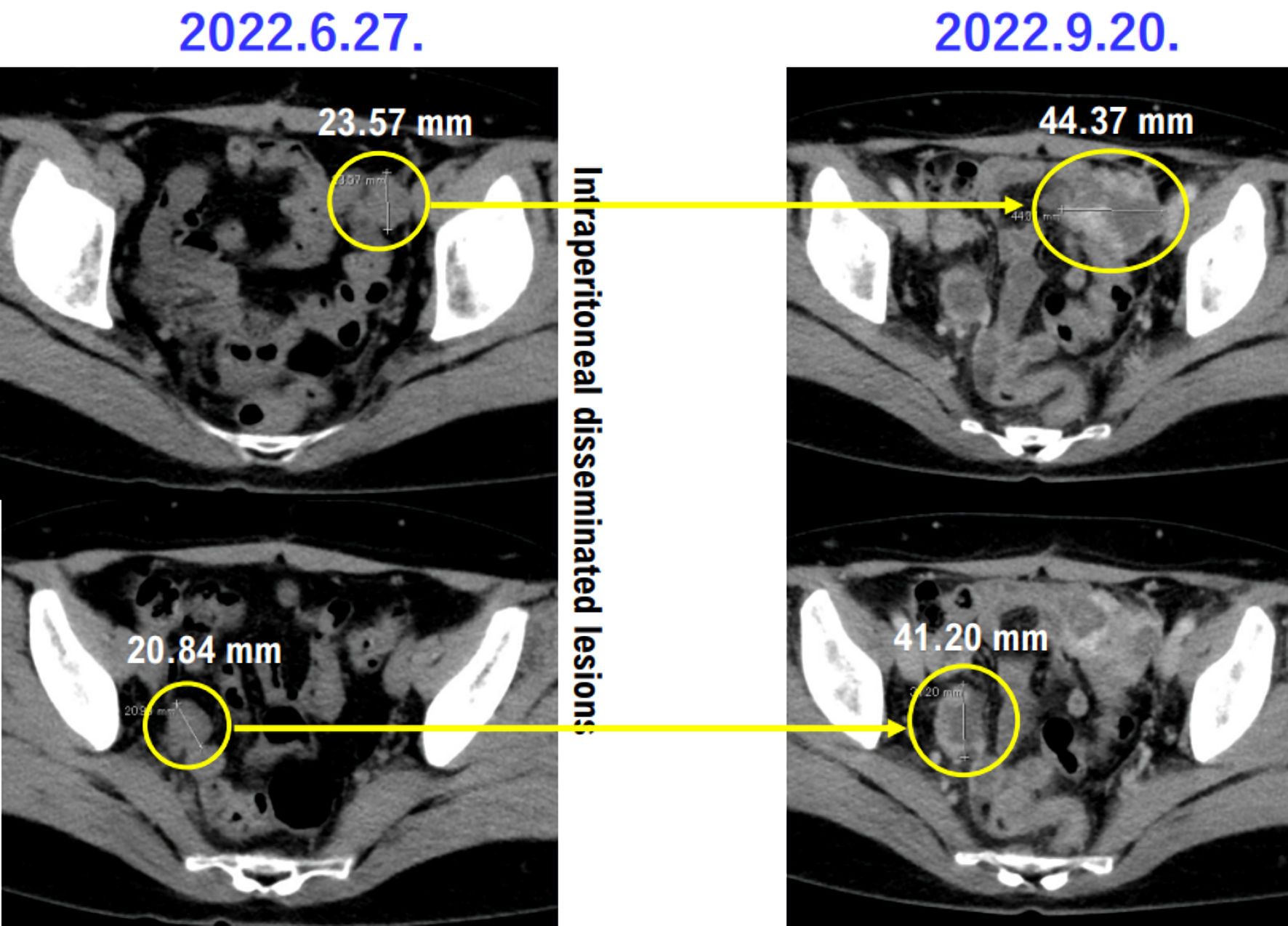 Click for large image | Figure 2. Contrast-enhanced CT showing intraperitoneal disseminated lesions in case 1 patient. Images of intra-abdominal disseminated lesions in case 1 patients taken by contrast-enhanced CT on June 27, 2023, and September 20, 2023, are presented. The lesions of the intraperitoneal disseminated lesions are worsening. Yellow circles indicate intraperitoneal disseminated lesions. CT: computed tomography. |
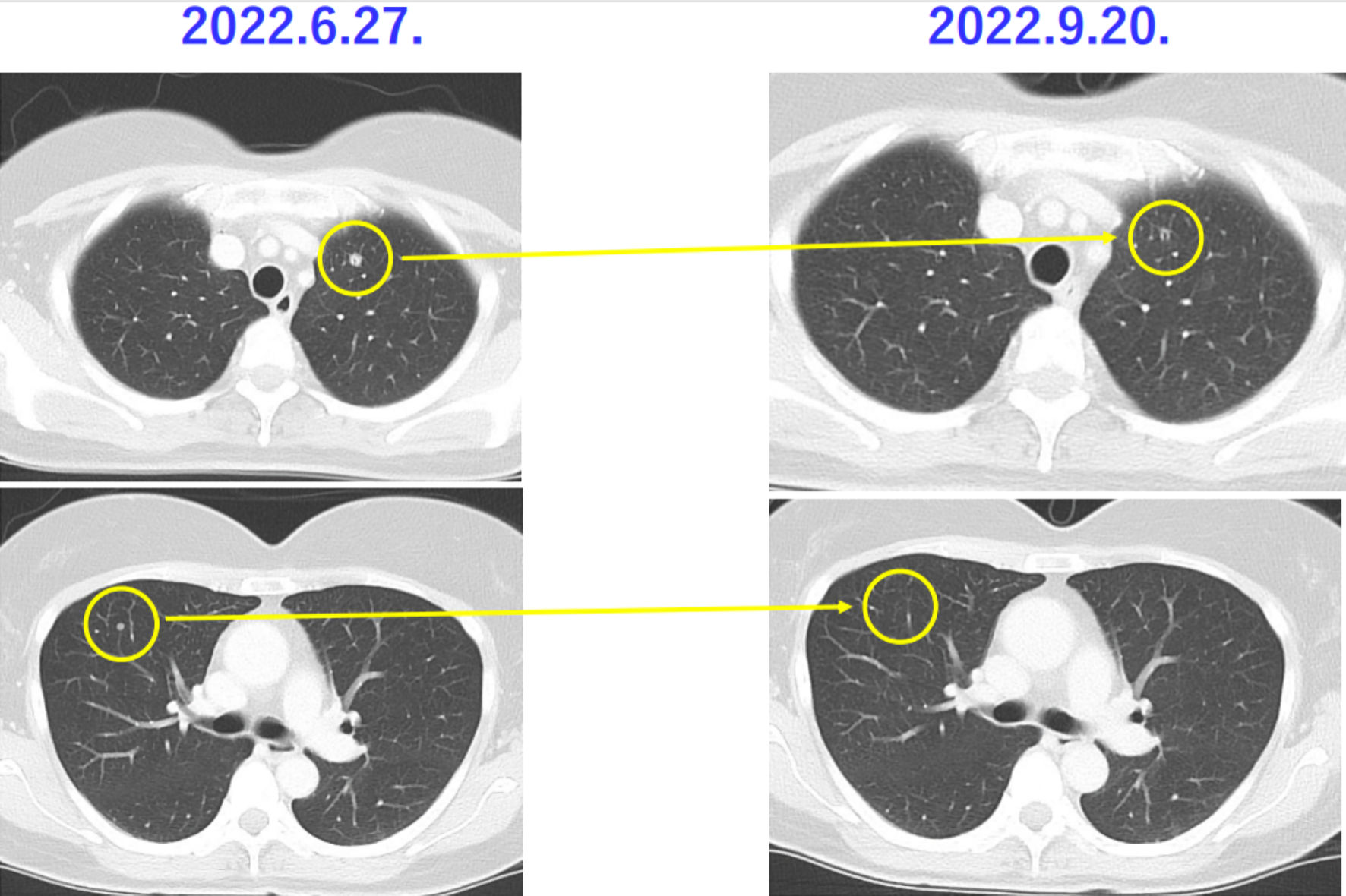 Click for large image | Figure 3. Chest CT showing metastatic lesions to the lungs of case 1 patient. Images of metastatic lesions to the lungs of case 1 patients taken by chest CT on June 27, 2023, and September 20, 2023 are presented. The lesions of metastatic lesions to the lungs are shrinking. Yellow circles indicate metastatic lesions to the lungs. CT: computed tomography. |
From July 11, 2022, to September 5, 2022, she received three courses of adriamycin (Kyowa Kirin Co., Ltd. Chiyoda, Tokyo, Japan) single agent. On September 15, 2022, hydronephrosis, which occurred owing to right ureter obstruction due to intraperitoneal lesion exacerbation, was observed. Enhance-contrasted CT revealed minimal lung metastatic lesions; however, as the intraperitoneal disseminated tumors were increasing in size, the treatment effect was judged as progressive disease (PD) by Response Evaluation Criteria in Solid Tumor (RECIST) (Figs. 2, 3). Therefore, the therapeutic drug was changed to pazopanib, a multikinase inhibitor that specifically inhibits vascular endothelial growth factor receptor signal, platelet-derived growth factor receptor signal, and c-Kit signal. Subsequently, cancer genome test (FoundationOne® CDx tissue) was performed (tumor content, 50%). The cancer genome test showed TMB-high of 14 Muts/Mb; therefore, pembrolizumab, an immune checkpoint inhibitor (ICI) covered by health insurance, was prescribed and administered to this patient with TMB-high as a companion diagnosis (Supplementary Material 1, www.jocmr.org) (Table 1).
 Click to view | Table 1. FoundationOne® CDx Tissue Testing Results for the 55-Year-Old Patient With Advanced and Recurrent Uterine Leiomyosarcoma |
Based on the FoundationOne® CDx tissue test results, MutS homolog 6 (MSH6) - F1088fs*5 (variant allele fraction (VAF)) (Supplementary Material 3, www.jocmr.org), 1.51%) and MutY DNA glycosylase (MUTYH) - splice site 892-2A>G (VAF, 50.32%) were detected as molecules that should be considered for hereditary cancer, that is, MUTYH-associated polyposis (MAP) (Table 1). F1088fs*5, which was detected as a PV in the MSH6 molecule, is a genetic mutation that induces Lynch syndrome onset [17]. However, as the VAF of MSH6 - F1088fs*5 was 1.51%, it was considered a somatic mutation (Table 1). Additionally, splice site 892-2A>G, which was detected as a PV in the MUTYH molecule, is a genetic mutation that induces MAP onset [18]. MUTYH - splice site 892-2A>G had a VAF of 50.32%; therefore, it was considered a germinal mutation (Supplementary Material 3, www.jocmr.org). However, as no patient or family member developed MAP, investigating the genetic cause of MUTYH was not required.
Case 2
Case 2 was an 82-year-old female with a PS score of 1, diagnosed with uterine sarcoma recurrence (epithelioid leiomyosarcoma), with a medical history of hypertension, left ear hearing loss, mitral regurgitation, and cystocele managed with pessary placement, along with a family history of a brother with kidney and lung cancer.
In April 2019, abdominal simple total hysterectomy, bilateral adnexectomy, partial omentectomy, and small mesenteric lesion resection were performed as the recommended surgical treatment.
From May 2019 to October 2019, docetaxel plus gemcitabine was administered to the patient (six cycles in total). In October 2020, contrast-enhanced CT was performed and revealed pelvic recurrence (Fig. 4). Therefore, pazopanib treatment was started. In January 2021, the treatment effect was judged as PD by RECIST owing to the increasing size of intraperitoneal disseminated tumors. In February 2022, DXR treatment was initiated for uterine leiomyosarcoma.
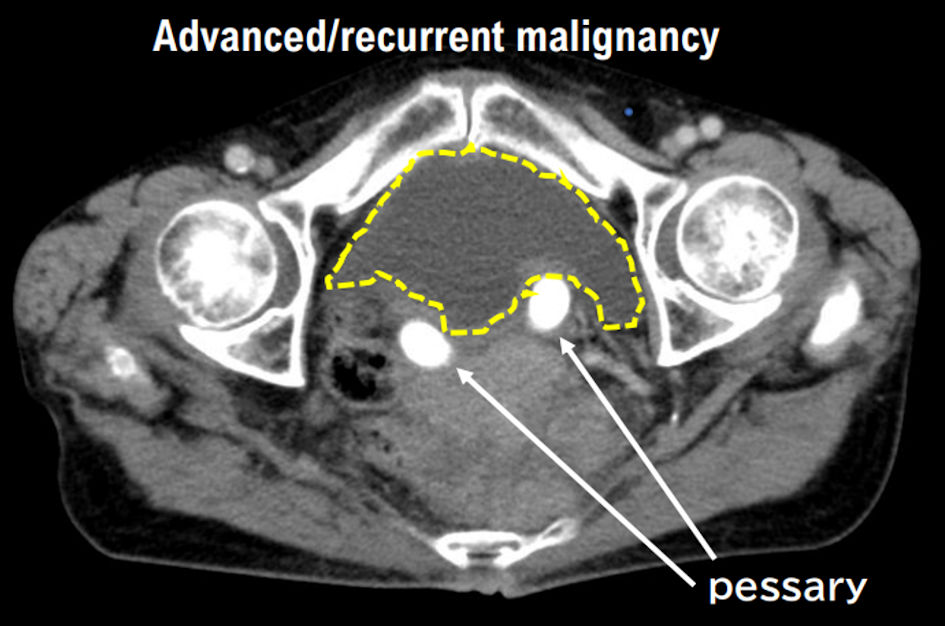 Click for large image | Figure 4. Progressive pelvic tumor of recurrent uterine leiomyosarcoma in case 2 patient by contrast-enhanced CT (October 6, 2022). Images of progressive pelvic tumor of recurrent uterine leiomyosarcoma in case 2 patient taken by contrast-enhanced CT are presented. The advanced pelvic tumor is getting worse. The yellow dashed line surrounds the advanced pelvic tumor. |
By August 2022, the eighth cycle of DXR was completed. As enhance-contrasted CT revealed that the intraperitoneal disseminated tumors were not decreasing in size, the treatment effect was judged as stable disease (SD) by RECIST. A cardiologist from the Department of Cardiovascular Medicine of our hospital performed cardiac function evaluation; although mitral regurgitation was noted, the cardiac function remained normal. In July 2022, tumor sections were shipped for cancer genome test (FoundationOne® CDx tissue) (tumor content, 80%). The results showed PVs in the serine-threonine kinase AKT molecule; therefore, the multikinase inhibitor pazopanib, which was covered by health insurance, was prescribed and administered to this patient (Table 2).
 Click to view | Table 2. FoundationOne® CDx Tissue Testing Results for the 82-Year-Old Patient With Advanced and Recurrent Uterine Leiomyosarcoma |
Based on the FoundationOne® CDx tissue test results, retinoblastoma protein 1 (RB1) - loss exons 18-27 (CN0) and tumor protein p53 (TP53) - G187V, K132N (VAF, 44.89%) were detected as molecules that should be considered for hereditary cancer (Table 2). Loss exons 18-27, detected as a PV in the RB1 molecule, is a germinal mutation that induces retinoblastoma development [19]. However, the copy number of RB1 - loss exons 18-27 was 0, and no retinoblastoma cases were observed under the age of 30 in the family; therefore, investigating the disease onset was not required (Table 2). The VAF of G187V and K132N, which were detected as PVs in TP53 molecule, was 44.89%; therefore, this TP53 mutation may be a germinal mutation that induces Li-Fraumeni syndrome onset [20]. However, as no family members with osteosarcoma, breast cancer, or soft tissue tumors under the age of 30 were noted, TP53 - G187V, K132N as a genetic cause of Li-Fraumeni syndrome was not considered. The FoundationOne® CDx tissue test results indicated that alpha-thalassemia/impaired intellectual X-linked (ATRX) and mediator complex subunit 12 (MED12) PVs were associated with hereditary cancer onset. However, based on the results of a clinical trial in a Japanese cohort, ATRX and MED12 PVs were not linked to hereditary cancer development in Japanese patients.
| Discussion | ▴Top |
Clinical trials using various antitumor agents are being conducted to establish treatments for uterine leiomyosarcomas; however, the effectiveness of various antitumor agents against uterine leiomyosarcomas is limited [21]. Under these circumstances, advances in cancer genomic medicine have revealed the effectiveness of PV-targeting antitumor agents, even for malignant tumors for which no treatment has been established to date [22]. This time, our medical staff experienced treatment with pembrolizumab or pazopanib for uterine leiomyosarcomas with TMB-high and uterine leiomyosarcomas with AKT PV detected through cancer genomic gene panel testing.
At present, the results of clinical trials have not shown the effectiveness of ICI against uterine leiomyosarcomas. This result may be because of the absence of biomarkers for ICI, including microsatellite instability-high (Supplementary Material 4, www.jocmr.org) and TMB-high, in the uterine leiomyosarcoma of the participants enrolled in the clinical trial [23]. Furthermore, in previous clinical trials, the completed efficacy of serine-threonine kinase inhibitors against uterine leiomyosarcomas has not been confirmed [24]. This result also suggests that biomarkers for kinase inhibitors are not present in the uterine leiomyosarcoma of the participants enrolled in the clinical trial. Personalized medical care for advanced and recurrent malignant tumors can prolong patient survival. In other words, in future cancer medicine, cancer genomic medicine using cancer genome gene panels will be essential in selecting antitumor agents for advanced and recurrent malignant tumors.
The National Cancer Institute (NCI) in the USA announced that a new initiative will evaluate the effectiveness of drug combinations for treating cancers with particular genetic changes [25]. In cancer genomic medicine in Japan, single-agent administration of already approved antitumor agents is prioritized for cases wherein PVs have been detected in multiple molecules in a single patient. Next, an antitumor agent that has been shown effective in clinical trials and case reports is administered as a single agent. To date, in cancer genomic medicine in Japan, in several cases, combination therapy with multiple antitumor agents has not been performed for patients with PVs in multiple molecules. In the future, cancer genomic medicine, such as in advanced or recurrent uterine leiomyosarcomas wherein PV in the breast cancer 1 (BRCA1) or BRCA2 gene and an active PV in AKT are detected using cancer genomic testing, combination therapy with a poly (ADP-ribose) polymerase (PARP) inhibitor (e.g., olaparib or niraparib) (Supplementary Material 5, www.jocmr.org) and a tyrosine kinase inhibitor should be considered [11, 26]. In Japan, prescriptions of the combination therapy of a PARP inhibitor with tyrosine kinase inhibitor for advanced and recurrent uterine leiomyosarcomas are not covered by health insurance. Therefore, selecting the antitumor agent depends on the results of clinical trials in other countries. NCI’s new initiative will be beneficial to cancer genomic medicine in Japan. Currently, in clinical trials, pharmaceutical companies are also examining the efficacy of combination therapy with multiple approved antitumor agents against various advanced and recurrent malignancies. The results of these clinical trials and NCI’s new initiative are significant for cancer genomic medicine development.
Advances in cancer genomic medicine contribute to prolonging the lives of patients with advanced and recurrent malignant tumors. In Japan, if combination therapy with multiple antitumor agents is covered by insurance on the basis of the cancer genome gene panel test results, it is believed that patients with advanced and recurrent malignant tumors will live longer. However, malignant tumor cells acquire resistance mechanisms to various antitumor agents; therefore, treatment using different antitumor agents is required. To elucidate the detailed mechanisms by which malignant tumors acquire resistance to antitumor agents, further basic medical and clinical research is required.
| Supplementary Material | ▴Top |
Suppl 1. Tumor mutational burden through cancer genome gene panel testing.
Suppl 2. The Eastern Cooperative Oncology Group Performance Status scale.
Suppl 3. The variant allele fraction through cancer genome gene panel testing.
Suppl 4. Microsatellite instability as biomarker for immune checkpoint inhibitor.
Suppl 5. Poly (ADP-ribose) polymerase inhibitor.
Acknowledgments
We thank all medical staff for clinical research at the Kyoto University Hospital and the National Hospital Organization Kyoto Medical Center.
Financial Disclosure
This clinical research was performed with research funding from the following: Japan Society for Promoting Science for TH (Grant No. 19K09840, 23K08881), Tokyo, Japan, and START-program Japan Science and Technology Agency for TH (Grant No. STSC20001), Tokyo, Japan and the National Hospital Organization Multicenter clinical study for TH (Grant No. 2019-Cancer in general-02), Tokyo, Japan, and the Japan Agency for Medical Research and Development (AMED) (Grant No. 22ym0126802j0001), Tokyo, Japan.
Conflict of Interest
The authors state no conflict of interest.
Informed Consent
We have obtained informed consent statements from people participating in clinical studies.
Author Contributions
All authors had full access to the data in the study and took responsibility for the integrity of the data and accuracy of the data analysis. TH and NK: research conduction. TH, NK, and KA: writing - original draft. TH and IK: writing - review and editing. IK: visualization. TH and IK: supervision. TH and IK: funding acquisition.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kotowski K, Malyszczak P, Towarek M, Jagasyk A, Murawski M, Sozanski R. An unusual coincidence of giant cervical leiomyoma and incidental ovarian granulosa cell tumor: A case report. Medicine (Baltimore). 2023;102(30):e34387.
doi pubmed pmc - Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149(1):3-9.
doi pubmed - Hartmann KE, Birnbaum H, Ben-Hamadi R, Wu EQ, Farrell MH, Spalding J, Stang P. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol. 2006;108(4):930-937.
doi pubmed - Moinfar F, Kremser ML, Man YG, Zatloukal K, Tavassoli FA, Denk H. Allelic imbalances in endometrial stromal neoplasms: frequent genetic alterations in the nontumorous normal-appearing endometrial and myometrial tissues. Gynecol Oncol. 2004;95(3):662-671.
doi pubmed - Cotangco K, Meram M, Lowe MP. Stabilization of metastatic uterine leiomyosarcoma using pembrolizumab. J Natl Compr Canc Netw. 2020;18(8):1012-1014.
doi pubmed - Wang YJ, Williams HR, Brzezinska BN, Gaidis A, Patel B, Munroe J, White J, et al. Use of pembrolizumab in MSI-high uterine leiomyosarcoma; a case report and review of the literature. Gynecol Oncol Rep. 2021;35:100701.
doi pubmed pmc - Murray SK, Clement PB, Young RH. Endometrioid carcinomas of the uterine corpus with sex cord-like formations, hyalinization, and other unusual morphologic features: a report of 31 cases of a neoplasm that may be confused with carcinosarcoma and other uterine neoplasms. Am J Surg Pathol. 2005;29(2):157-166.
doi pubmed - Nishikawa S, Hayashi T, Amano Y, Yaegashi N, Abiko K, Konishi I. Characteristic of concurrent uterine lipoleiomyoma and hemangioma by algorithm of candidate biomarkers for uterine mesenchymal tumor. Diagnostics (Basel). 2022;12(10):2468.
doi pubmed pmc - Tamura S, Hayashi T, Abiko K, Konishi I. Potential biomarkers associated with the nature of uterine benign mesenchymal tumors. J Clin Med Res. 2022;14(3):142-145.
doi pubmed pmc - Hensley ML, Miller A, O'Malley DM, Mannel RS, Behbakht K, Bakkum-Gamez JN, Michael H. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33(10):1180-1185.
doi pubmed pmc - Ingham M, Allred JB, Chen L, Das B, Kochupurakkal B, Gano K, George S, et al. Phase II study of olaparib and temozolomide for advanced uterine leiomyosarcoma (NCI Protocol 10250). J Clin Oncol. 2023;41(25):4154-4163.
doi pubmed - Hayashi T, Konishi I. Prospects and problems of cancer genome analysis for establishing cancer precision medicine. Cancer Invest. 2019;37(9):427-431.
doi pubmed - Hensley ML, Chavan SS, Solit DB, Murali R, Soslow R, Chiang S, Jungbluth AA, et al. Genomic landscape of uterine sarcomas defined through prospective clinical sequencing. Clin Cancer Res. 2020;26(14):3881-3888.
doi pubmed pmc - Dziadziuszko R, Hung T, Wang K, Choeurng V, Drilon A, Doebele RC, Barlesi F, et al. Pre- and post-treatment blood-based genomic landscape of patients with ROS1 or NTRK fusion-positive solid tumours treated with entrectinib. Mol Oncol. 2022;16(10):2000-2014.
doi pubmed pmc - Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104.
doi pubmed pmc - Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, Borghaei H, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992-1000.
doi pubmed pmc - Walker R, Clendenning M, Joo JE, Xue J, Mahmood K, Georgeson P, Como J, et al. A mosaic pathogenic variant in MSH6 causes MSH6-deficient colorectal and endometrial cancer in a patient classified as suspected Lynch syndrome: a case report. Fam Cancer. 2023;22(4):423-428.
doi pubmed pmc - Villy MC, Warcoin M, Filser M, Buecher B, Golmard L, Suybeng V, Schwartz M, et al. First report of medulloblastoma in a patient with MUTYH-associated polyposis. Neuropathol Appl Neurobiol. 2023;49(4):e12929.
doi pubmed - Vural O, Atalay HT, Kayhan G, Tarlan B, Oral M, Okur A, Pinarli FG, et al. Clinical and genetic characteristics of retinoblastoma patients in a single center with four novel RB1 variants. Int J Ophthalmol. 2023;16(8):1274-1279.
doi pubmed pmc - Sanchez-Heras AB, Ramon YCT, Pineda M, Aguirre E, Grana B, Chirivella I, Balmana J, et al. SEOM clinical guideline on heritable TP53-related cancer syndrome (2022). Clin Transl Oncol. 2023;25(9):2627-2633.
doi pubmed pmc - Lacuna K, Bose S, Ingham M, Schwartz G. Therapeutic advances in leiomyosarcoma. Front Oncol. 2023;13:1149106.
doi pubmed pmc - Kodama M, Shimura H, Tien JC, Newberg JY, Kodama T, Wei Z, Rangel R, et al. Sleeping beauty transposon mutagenesis identifies genes driving the initiation and metastasis of uterine leiomyosarcoma. Cancer Res. 2021;81(21):5413-5424.
doi pubmed - Ros J, Baraibar I, Saoudi N, Rodriguez M, Salva F, Tabernero J, Elez E. Immunotherapy for colorectal cancer with high microsatellite instability: the ongoing search for biomarkers. Cancers (Basel). 2023;15(17):4245.
doi pubmed pmc - Dickson MA, Mahoney MR, Tap WD, D'Angelo SP, Keohan ML, Van Tine BA, Agulnik M, et al. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol. 2016;27(10):1855-1860.
doi pubmed pmc - Harris E. NCI will study drug combinations in new precision treatment initiative. JAMA. 2023;330(1):13.
doi pubmed - Schram AM, Colombo N, Arrowsmith E, Narayan V, Yonemori K, Scambia G, Zelnak A, et al. Avelumab plus talazoparib in patients with BRCA1/2- or ATM-altered advanced solid tumors: results from JAVELIN BRCA/ATM, an open-label, multicenter, Phase 2b, tumor-agnostic trial. JAMA Oncol. 2023;9(1):29-39.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


