| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 2, February 2023, pages 116-126
Symptom Profile of Patients With Post-COVID-19 Conditions and Influencing Factors for Recovery
Norihiro Matsuokaa, c, Takuo Mizutania, Koji Kawakamib
aJyoto Hospital, 2-11-22, Hanaten-higashi, Tsurumi-ku, Osaka-shi, Osaka 538-0044, Japan
bDepartment of Pharmacoepidemiology, Graduate School of Medicine and Public Health, Kyoto University, Kyoto, Japan
cCorresponding Author: Norihiro Matsuoka, Jyoto Hospital, 2-11-22, Hanaten-higashi, Tsurumi-ku, Osaka-shi, Osaka 538-0044, Japan
Manuscript submitted December 11, 2022, accepted February 16, 2023, published online February 28, 2023
Short title: Factors of Persistence of Post-COVID-19 Conditions
doi: https://doi.org/10.14740/jocmr4855
| Abstract | ▴Top |
Background: The aim of the study was to examine the factors that influence the improvement of post-coronavirus disease 2019 (COVID-19) symptoms.
Methods: We investigated the biomarkers and post-COVID-19 symptoms status of 120 post-COVID-19 symptomatic outpatients (44 males and 76 females) visiting our hospital. This study was a retrospective analysis, so we analyzed the course of symptoms only for those who could follow the progress of the symptoms for 12 weeks. We analyzed the data including the intake of zinc acetate hydrate.
Results: The main symptoms that remained after 12 weeks were, in descending order: taste disorder, olfactory disorder, hair loss, and fatigue. Fatigue was improved in all cases treated with zinc acetate hydrate 8 weeks later, exhibiting a significant difference from the untreated group (P = 0.030). The similar trend was observed even 12 weeks later, although there was no significant difference (P = 0.060). With respect to hair loss, the group treated with zinc acetate hydrate showed significant improvements 4, 8, and 12 weeks later, compared with the untreated group (P = 0.002, P = 0.002, and P = 0.006).
Conclusion: Zinc acetate hydrate may improve fatigue and hair loss as symptoms after contracting COVID-19.
Keywords: COVID-19; SARS-CoV-2; Post-COVID-19 conditions; Fatigue; Hair loss; Sequela
| Introduction | ▴Top |
It has been reported that some patients with coronavirus disease 2019 (COVID-19) experienced the persistence of such symptoms as headache, fatigue, and/or taste/olfactory disorder, even after major symptoms were improved and infectivity disappeared under what are called post-COVID-19 conditions [1].
Huang et al reported that 76% of the COVID-19 patients experienced at least a persistent symptom 6 months after the onset of symptoms, 63% suffered fatigue or muscle weakness, and 23% had symptoms of anxiety/depression [2]. The systematic review of 45 papers reporting results of research in 9,751 cases by Nasserie et al [3] confirmed the development of such symptoms as fatigue or exhaustion (40.0%), shortness of breath or dyspnea (36.0%), anosmia (23.6%), taste disorder (15.6%), and anxiety and/or depression (14.9%).
Such persistent symptoms varied by state of continuation as well as respective incidence. Tan et al [4] reported that 74.1% of the patients showing olfactory disorder recovered by day 30, while some did not recover even 180 days later and that the median period for recovery was 14.9 days. Another study reported that fatigue persisted for several weeks to months, and fatigue lingered in 10-35% of the patients even after 6 months [5].
In Japan, three research teams were formed to investigate post-COVID-19 conditions, and the results were reported [6]. The more common lingering symptoms were fatigue, breathlessness, muscular weakness, and decline in concentration, and the rates of persistence in 3 months were 21%, 14%, 12%, and 11%, respectively.
On the other hand, there are scarce data on characteristics of patients showing or suffering persistent symptoms. There is only a poor understanding of post-COVID-19 conditions, while risk factors such as being of the female sex, smoking, and obesity, and the risk of long-term persistence increase inversely with age [7].
We previously reported that the risk of death rose in inpatients with COVID-19 when the serum zinc (Zn) concentration at hospitalization was lower than 54 µg/dL [8]. We have prescribed supplement of zinc to the outpatients with post-COVID-19 conditions as necessary. We supposed that zinc acetate hydrate was effective in improving symptoms in our patient examinations. We did a detailed analysis of the most common post-COVID-19 conditions: fatigue, taste disorder, olfactory disorder, and hair loss.
After examining the factors affecting recovery from post-COVID-19 conditions, including zinc acetate hydrate, and understanding the factors we found in our clinical practice, we sought a way to ensure quicker recovery.
| Materials and Methods | ▴Top |
Study design
The study was conducted as a single-institutional descriptive epidemic study. We employed the opt-out policy for use of the existing data. Having gone through a review and obtained an approval of the ethics review board on August 3, 2022, we performed the study in accordance with the Declaration of Helsinki and Ethical Guidelines for Medical and Biological Research Involving Human Subjects.
Subjects and treatment
We analyzed patients who had been followed up until 12 weeks after, designating as a population those who had visited our outpatient department with post-COVID-19 conditions as a main complaint. The study period started on August 1, 2021 and ended on June 1, 2022. The analysis only included the existing data; there were no exclusion criteria. General practitioners were in charge of the examination. We confirmed the presence or absence of symptoms in patients using a self-reported check sheet (original sheet) at the time of medical interview (Supplementary Material 1, www.jocmr.org). According to World Health Organization, a “post-COVID-19 condition occurs in individuals with a history of probable or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.” We analyzed all post-morbid symptoms, however, regardless of symptom duration. Regarding hair loss, we used a loupe to check the condition of the scalp. For patients with hypozincemia, we prescribed 50 mg of zinc acetate hydrate tablets to be taken twice a day and continued until the patient healed. (In Japan, doctors cannot prescribe zinc acetate hydrate because it is not covered by insurance unless it is hypozincemia.)
Data collected
Data collected included: age, sex, presence of post-COVID-19 conditions (fatigue, dyspnea, cough/sputum, decline in cognitive dysfunction/concentration, headache, diarrhea, taste disorder, olfactory disorder, hair loss, and depression), improvement of such symptoms in 4, 8, and 12 weeks after the onset; total protein, albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), cholinesterase (ChE), lipase, uric acid, estimated glomerular filtration rate (eGFR), triglyceride, low-density lipoprotein (LDL) cholesterol, blood glucose, Na, Cl, K, Ca, P, Mg, Zn, Cu (determined as serum levels respectively); C-reactive protein (CRP), white blood cell (WBC), hemoglobin, platelet (PLT), vitamin B2, vitamin B12, free thyroxine (FT4), thyroid-stimulating hormone (TSH) (determined as blood levels respectively); use of zinc acetate hydrate, use of mecobalamin, use of mecobalamin injections at the first visit for post-COVID-19 conditions.
Endpoints
Because of the study design as a descriptive epidemic study, we performed an exploratory investigation on the following items without using any primary endpoints: 1) Age, sex, and status of follow-up of patients with post-COVID-19 conditions; 2) Status of improvement of post-COVID-19 conditions 4, 8, and 12 weeks later (for the followed-up cases only); 3) Relationship between the use of zinc acetate hydrate and the status of improvement of symptoms in the patients showing fatigue/taste disorder/olfactory disorder/hair loss (for the followed-up cases only); 4) Factorial analysis of the status of improvement (age, sex, treatment, laboratory test values at the first outpatient visit (abbreviated below as “first-visit test values”)) in the patients showing fatigue/taste disorder/olfactory disorder/hair loss (for the followed-up cases only).
Statistical analysis
Summary statistics of patient backgrounds are indicated as frequency (n) and percentage (%) or mean ± standard deviation. The patients were grouped into two by use of zinc acetate hydrate. Fisher’s exact test was used for comparison in the status of improvement of post-COVID-19 conditions. Wald Chi-squared test was used for analyzing improvement factors for fatigue, taste disorder, olfactory disorder, and hair loss. We used the items with P < 0.25 in univariate analysis in the Wald Chi-squared test for multivariate analysis. (If there were many applicable explanatory variables, only items with P < 0.1 were included.) Analysis used R analytics, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria [9]). All statistical tests employed the two-tailed significance level of 5%.
| Results | ▴Top |
The statistical analysis of the study involved 120 cases consisting of 44 males (36.7%) and 76 females (63.3%). Table 1 shows patient backgrounds of those showing post-COVID-19 conditions. Numbers of cases showing symptoms (duplicated cases included) are 56 cases for fatigue, six for dyspnea, 28 for cough/sputum, 10 for decline in cognitive dysfunction/concentration, 35 for headache, 20 for diarrhea, 77 for taste disorder, 70 for olfactory disorder, 42 for hair loss, and eight for depression (Table 1). The Zn level was determined as 83.7 ± 17.4 mg/dL in the outpatients (Table 2), and all 53 cases (44.2%) with the levels below 80 mg/dL were found to be users of prescribed zinc acetate hydrate. Looking at the use of the agents that can affect improvement of post-COVID-19 conditions, we noted that 50 cases used mecobalamin and eight cases took mecobalamin injections.
 Click to view | Table 1. Patient Profile and Follow-Up Status in Treatment of Post-COVID-19 Conditions |
 Click to view | Table 2. The First-Visit Test Values in All Patients |
Changes in improvement rates of symptoms are given in a chronological order (Fig. 1). Symptoms of dyspnea were improved in the patients except for a case 4 weeks later, and all patients experienced symptomatic improvement 8 weeks later. On the other hand, symptoms of cough/sputum remained in 33.3% of the patients until week 4; 12 weeks later, the symptoms were improved in 92.6% of the patients. Diarrhea symptoms were improved in 87.5% of the patients 4 weeks later, and 93.8% 8 weeks later. Taste disorder persisted in 39.2% of the patients 4 weeks after and 20.3% even 12 weeks after. Symptoms of olfactory disorder lasted in 41.8% of the patients until 4 weeks later and 25.4% until 12 weeks later. Hair loss persisted in 41.0% of the patients 4 weeks later and 15.4% 12 weeks later.
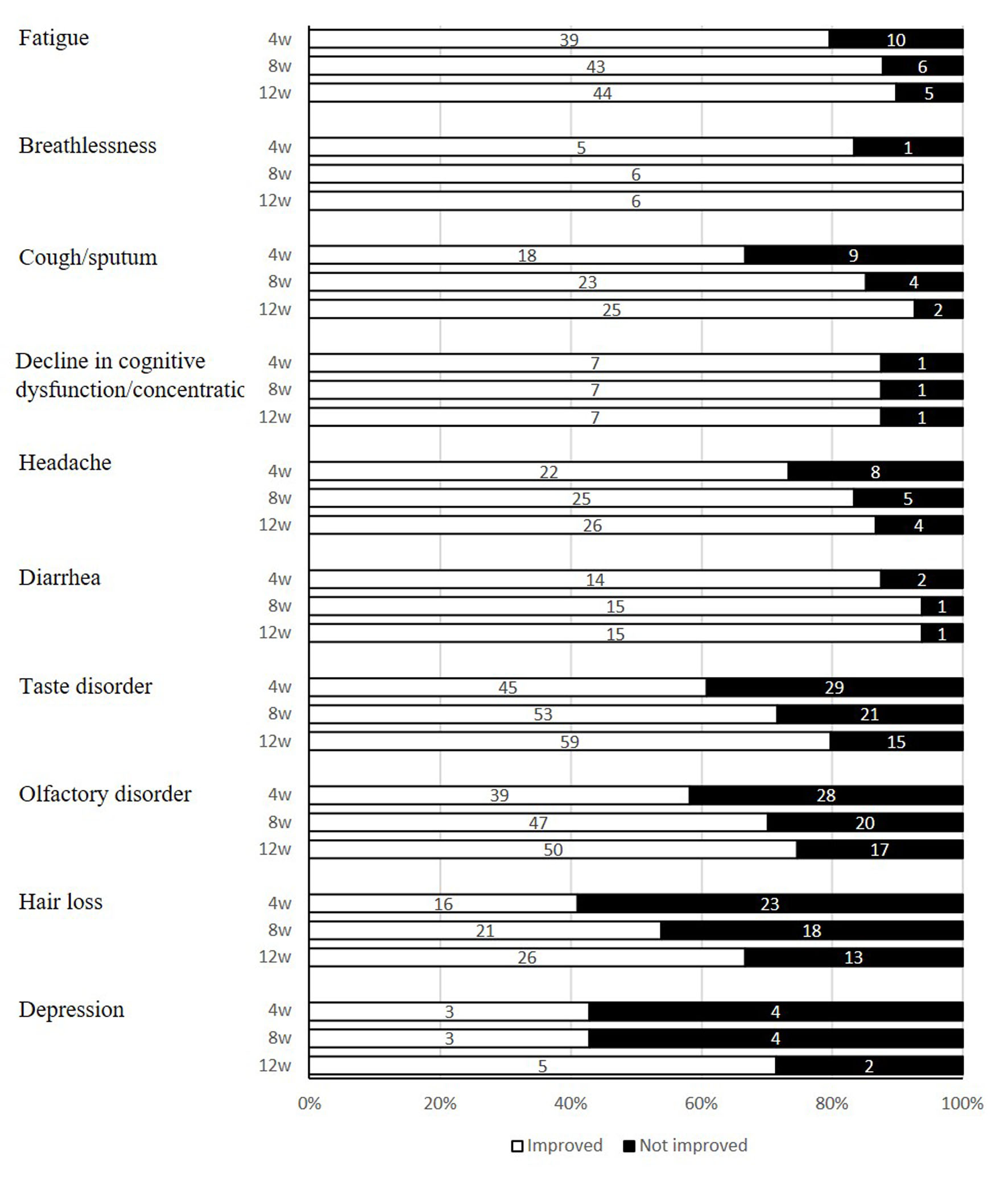 Click for large image | Figure 1. Improvement status of post-COVID-19 conditions. COVID-19: coronavirus disease 2019. |
We examined the use of pharmacotherapy with zinc acetate hydrate and the status of improvement of symptoms in the patients showing fatigue/taste disorder/hearing disorder/hair loss that were more commonly observed (Fig. 2). Fatigue was improved in all cases treated with zinc acetate hydrate 8 weeks later, exhibiting a significant difference from the untreated group (P = 0.030). The similar trend was observed even 12 weeks later, although there was no significant difference (P = 0.060). As for taste disorder and olfactory disorder, no significant difference was found in the use of zinc acetate hydrate. With respect to hair loss, the group treated with zinc acetate hydrate showed significant improvements 4, 8, and 12 weeks later, compared with the untreated group (P = 0.002, P = 0.002, and P = 0.006).
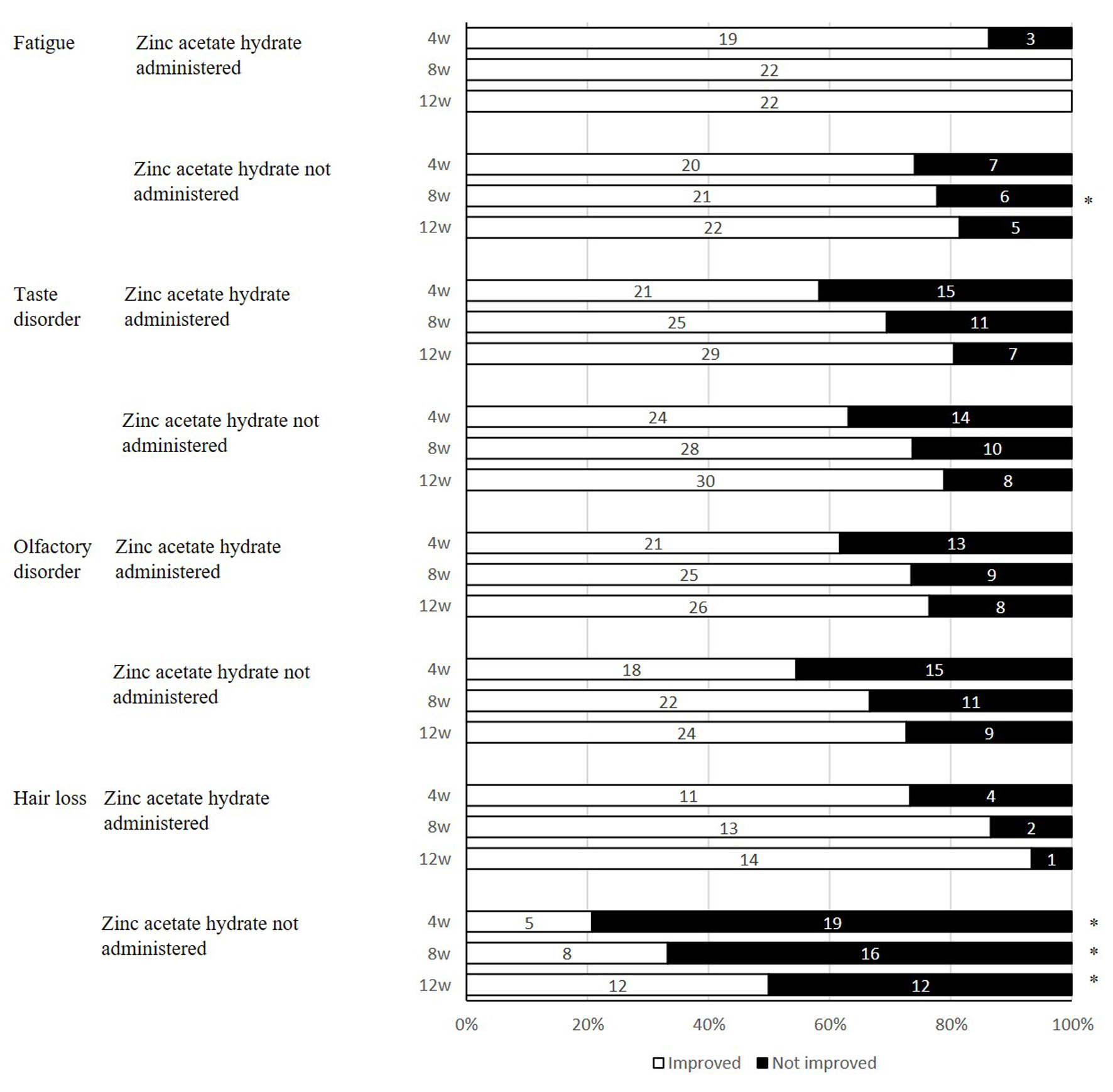 Click for large image | Figure 2. The use of zinc acetate hydrate and improvement status of post-COVID-19 conditions after 12 weeks. *P < 0.05, comparison with use of zinc acetate hydrate. Fisher’s exact test. COVID-19: coronavirus disease 2019. |
Tables 3 and 4 summarize univariate analysis results of improvement factors of fatigue, taste disorder, hearing disorder, and hair loss 12 or 4 weeks later, and Table 5 shows multivariate analysis results. None of the patients who used zinc acetate hydrate remained symptomatic of fatigue after 12 weeks, and only one patient remained symptomatic of hair loss after 12 weeks. These were not suitable for factorial analysis after 12 weeks because it was too biased. We therefore focused on remaining symptoms after 4 weeks and performed a multivariate analysis of the factors. As for hair loss, high potassium levels and treatment with zinc acetate hydrate were found to be significant improvement factors. In addition, as a result of performing the same analysis by converting to binary values above and below the median instead of actually measured values of potassium, potassium was no longer a significant factor (Supplementary Material 2, www.jocmr.org). We also performed a multivariate analysis with the same explanatory variables for each symptom and found no significant factors (Supplementary Material 3, www.jocmr.org).
 Click to view | Table 3. Analysis Results of Improvement Factors After 12 Weeks for Post-COVID-19 Conditions |
 Click to view | Table 4. Analysis Results of Improvement Factors After 4 Weeks for Post-COVID-19 Conditions |
 Click to view | Table 5. Multivariate Analysis Results for Improvement Factors of Symptoms 4 or 12 Weeks Later |
| Discussion | ▴Top |
Our study found taste disorder, olfactory disorder, fatigue, and hair loss occurred more commonly in our outpatients undergoing treatment of post-COVID-19 conditions (Table 1). It is difficult to compare precisely our study to the report [6] by the research team of the Ministry of Health, Labour and Welfare (MHLW). The subjects of our study were the patients visiting us for their experience of post-COVID-19 conditions. They might experience more symptoms that interfere with daily life than those studied by the MHLW team, which followed up patients who had symptoms at the time of discharge from hospital. This suggests that more patients may seek medical assistance due to taste disorder and/or olfactory disorder than for tiredness/fatigue, difficulty breathing, sleep disorder, and/or decline in cognitive dysfunction/concentration despite the lower incidence.
In our study, the number of female patients (63.3%) was greater than male (36.7%). Taking into account the results of our study and the past research report [7, 10], we surmise that women may be prone to such symptoms. These, however, are insufficient to ascertain whether there is a gender difference and, if so, its cause. A large-scale epidemiological survey will be required.
Other researchers reported long-lasting sequelae [11-13]. In our study, symptoms remained even 12 weeks after the development of post-COVID-19 conditions in some cases. Over 20% of the patients still suffered from taste disorder and/or olfactory disorder (Fig. 2). Such patients would require follow-up, including checking for zinc.
We previously reported that inpatients with COVID-19 showed the higher risk of death when serum Zn level was lower than 54 µg/dL at hospitalization [7]. We also focused on zinc intake in the study, considering that many researchers had reported that COVID-19 patients were likely to have lower serum Zn level [14]. In our study, we administered zinc acetate hydrate to all of the cases with hypozincemia. That is, the division of patients into two groups by use of zinc acetate hydrate can correspond to presence of hypozincemia at the start of the study. We therefore examined the difference in course of post-COVID-19 conditions in two groups differentiated by the administration of zinc acetate hydrate, since the initial test values of Zn were not suitable for factorial analysis. As a result, zinc acetate hydrate was found to improve fatigue and hair loss significantly (Fig. 2).
Past research reported that zinc deficiency was closely related to these long-term effects of COVID-19 fatigue, taste disorder and olfactory disorder [14]. It was previously reported that the mean recuperative period was significantly shorter in patients treated with zinc sulfate than patients without using zinc sulfate [15], the low zinc status caused taste disorder [16], and zinc products improved taste disorder [17-19]. Our study, however, did not find treatment with zinc acetate hydrate a predictor for improvement of taste disorder and olfactory disorder. Organs of taste and olfactories are sensory organs. Some people, like wine stewards, have well-developed senses, while others do not. There is a study reporting that olfactory stimulation and other training may help restore olfactory sense [20]. Zinc supplementation alone may not be effective enough for improvement. On the other hand, it may have been easier for patients to perceive improvement of fatigue under the use of zinc supplementation because, unlike abnormalities in local sensory organs, the symptom may have caused fewer individual differences in recovery. For improvement of hair loss as well, improvement may have been perceived more easily than taste and olfactory sense because physicians assessed the symptom objectively, that is, without having to refer to patients’ feelings.
We did not use results for fatigue for factorial analysis 12 weeks after because 8 weeks after, all of the cases recovered from the symptom in the group treated with zinc acetate hydrate. As a reference, we performed a factorial analysis on the improvement after 4 weeks, but zinc acetate hydrate was not a significant factor. In addition, although there were items that were significant factors in univariate analysis, there were none that were significant factors in multivariate analysis. It was suggested that the improvement effect of zinc acetate hydrate may not be obtained in 4 weeks, but it may be effective in 8 weeks or more. Fatigue can develop due to metabolic change induced by zinc deficiency [21]. A study reported that zinc supplementation helped improve fatigue [22].
Our study suggests that zinc supplementation may improve hair loss that had been developed after COVID-19. Previous studies had reported that zinc deficiency led to hair loss [23-25]. Zinc supplementation, therefore, may be a useful option in future treatment of post-COVID-19 conditions.
It has been confirmed that in COVID-19 patients, zinc reduces angiotensin converting enzyme-2 (ACE-2) receptor expression and inhibits RNA-dependent RNA polymerase of SARS-CoV-2 to prevent SARS-CoV-2 from invading in cells [26]. Zinc prevents cytokine storm occurring after invasion of SARS-CoV-2 in cells through its anti-inflammatory activity [26].
There are many different factors of zinc deficiency [27]. In addition to geographical, social and nutritional factors, one of the other factors is a consequence of viral infection. As zinc supplementation may drastically decrease natural immunity and adaptive antiviral immunity [28, 29], early supplementation would be preferable before it becomes a vicious spiral.
In addition, multivariate analysis of hair loss improvement factors after 4 weeks showed that the measured value of potassium was one of the factors, but it became insignificant when replaced with an explanatory variable divided into two by the median value. From this we deduced that the influence of outliers was large. Also, even in multivariate analysis at 4 weeks, the odds ratio of using zinc acetate hydrate is quite high. It is hoped that reports from studies based on more cases will be published in the future.
Limitations to the study
As a single-institutional descriptive epidemic study, our study only involved the patients having persistent post-COVID-19 conditions, excluding those who did not. We were unable to calculate the sample size and follow up patients who had stopped visiting us. Additionally, we prescribed zinc acetate hydrate, but we could not rigorously check its adherence status. We did not use internationally standardized quality of life (QOL) indices for assessing improvement of post-COVID-19 conditions in cases where patient self-assessment was partly employed. We cannot mention the course of test values because our factorial analysis only employed initial test results. We cannot describe the effects of zinc on improvement as we can for first-visit test values of zinc and the course of changes because we administered zinc acetate hydrate to all of cases of hypozincemia showing Zn values less than 80 mg/dL, and we were not allowed to determine zinc levels every time.
We would like to establish therapies by accumulating data on long-term outcomes in the patients with lingering symptoms and data on effects of drugs and continuously examining the factors affecting post-COVID-19 conditions.
Conclusions
In our outpatients currently under treatment of post-COVID-19 conditions, we found olfactory disorder, taste disorder, hair loss, and fatigue still prevalent even 12 weeks after development. Zinc acetate hydrate improved fatigue and hair loss. Our results suggest that zinc acetate hydrate may be a useful treatment for fatigue and hair loss developed as post-COVID-19 conditions.
| Supplementary Material | ▴Top |
Suppl 1. A self-reported check sheet (original sheet) at the time of medical interview.
Suppl 2. Multivariate analysis results for improvement factors of hair loss 4 weeks later (other patterns).
Suppl 3. Multivariate analysis results for improvement factors of symptoms 4 or 12 weeks later (other patterns).
Acknowledgments
None to declare.
Financial Disclosure
The authors have no funding source to disclose concerning this report.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Informed consent was not required for this study, because this is a retrospective study. We provided an opt-out document in Jyoto Hospital to allow the study subjects to decline the use of their data.
Author Contributions
NM conceived and designed the experiments, analyzed the data, wrote the first draft of the manuscript, and contributed to acquisition and interpretation of data and reviewed/edited the manuscript. TM contributed to the interpretation of data and reviewed/edited the manuscript. KK contributed to the study concept and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615.
doi pubmed - Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232.
doi pubmed - Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417.
doi pubmed - Tan BKJ, Han R, Zhao JJ, Tan NKW, Quah ESH, Tan CJ, Chan YH, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ. 2022;378:e069503.
doi pubmed - Sandler CX, Wyller VBB, Moss-Morris R, Buchwald D, Crawley E, Hautvast J, Katz BZ, et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis. 2021;8(10):ofab440.
doi pubmed - COVID-19 Control Advisory Board of the Ministry of Health, Labour and Welfare. Research for understanding the actual conditions of long-term complications of the COVID-19 and elucidating the pathophysiology. (in Japanese) [Internet. [cited Sep 19, 2022]. Available from: https://www.mhlw.go.jp/content/10900000/000945990.pdf.
- Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, Taverner T, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714.
doi pubmed - Donoso-Navarro E, Arribas Gomez I, Bernabeu-Andreu FA. IL-6 and Other Biomarkers associated with Poor Prognosis in a Cohort of Hospitalized Patients with COVID-19 in Madrid. Biomark Insights. 2021;16:11772719211013363.
doi pubmed - https://www.R-project.org/.
- Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, Boscolo-Rizzo P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089-2090.
doi pubmed - Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines Corona Research I. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452-461.
doi pubmed - Kim Y, Bitna H, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. 2022;22(1):93.
doi pubmed - Goertz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542-2020.
doi pubmed - Marumo S. Aftereffects of COVID-19 and its medical treatment. (in Japanese) J-IDEO. 2022;6(2):188-198.
- Abdelmaksoud AA, Ghweil AA, Hassan MH, Rashad A, Khodeary A, Aref ZF, Sayed MAA, et al. Olfactory disturbances as presenting manifestation among egyptian patients with COVID-19: possible role of zinc. Biol Trace Elem Res. 2021;199(11):4101-4108.
doi pubmed - Ikeda M, Ikui A. Dysgeusia and zinc deficiency. (in Japanese) Biomed Res Trace Elements. 2007;18(1):10-14.
- Yoshida S, Endo S, Tomita H. A double-blind study of the therapeutic efficacy of zinc gluconate on taste disorder. Auris Nasus Larynx. 1991;18(2):153-161.
doi pubmed - Sakai F, Yoshida S, Endo S, Tomita H. Double-blind, placebo-controlled trial of zinc picolinate for taste disorders. Acta Otolaryngol Suppl. 2002;122(4):129-133.
doi pubmed - Tanaka M, Oki Y, Katano H, Shoda Y, Ohima T. The study of administration of zinc acetate hydrate for taste disorder with hypozincemia (in Japanese). Journal of Zinc Nutritional Therapy. 2020;10(2):82-87.
- Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119(3):496-499.
doi pubmed - Mills CF. Zinc in human biology. London; New York: Springer-Verlag; 1988.
- Yalcin SS, Engur-Karasimav D, Alehan D, Yurdakok K, Ozkutlu S, Coskun T. Zinc supplementation and TNF-alpha levels in vaccinated cardiac patients. J Trace Elem Med Biol. 2011;25(2):85-90.
doi pubmed - Muhamed PK, Vadstrup S. Zinc is the most important trace element (in Danish). Ugeskr Laeger. 2014;176(5):V11120654..
- Saper RB, Rash R. Zinc: an essential micronutrient. Am Fam Physician. 2009;79(9):768-772.
- Ogawa Y, Kinoshita M, Shimada S, Kawamura T. Zinc and skin disorders. Nutrients. 2018;10(2):199.
doi pubmed - Imran M, Fatima W, Alzahrani AK, Suhail N, Alshammari MK, Alghitran AA, Alshammari FN, et al. Development of therapeutic and prophylactic zinc compositions for use against COVID-19: a glimpse of the trends, inventions, and patents. Nutrients. 2022;14(6):1227.
doi pubmed - Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, Sahoo S, et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res. 2021;199(8):2882-2892.
doi pubmed - Gupta S, Read SA, Shackel NA, Hebbard L, George J, Ahlenstiel G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells. 2019;8(6):603.
doi pubmed - Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv Nutr. 2019;10(4):696-710.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


