| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Short Communication
Volume 14, Number 11, November 2022, pages 487-491
Alterations in Laboratory Parameters Related to Disease Severity in Vaccinated Patients Against SARS-CoV-2
Maria Lagadinoua, b, c, George Eleftherakisa, Dimitris Papageorgioua, Anastasia Chionia, Themistoklis Paraskevasa, Christina Platanakia, Markos Marangosa, Dimitrios Velissarisa
aDepartment of Internal Medicine, University Hospital of Patras, Patras, Greece
bDepartment of Nursing, University of Patras, Patras, Greece
cCorresponding Author: Maria Lagadinou, Department of Nursing, University of Patras, Patras, Greece
Manuscript submitted September 3, 2022, accepted October 26, 2022, published online November 29, 2022
Short title: COVID-19 Vaccines and Blood Biomarkers
doi: https://doi.org/10.14740/jocmr4821
| Abstract | ▴Top |
Background: Coronavirus disease 2019 (COVID-19) has spread rapidly worldwide with global financial and health care systems consequences. It is already well recognized that immunization against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a precondition for blocking mutations and prevent the emergence of variants. The aim of the study was to investigate the possible relationship between COVID-19 vaccines and the commonly used disease-related blood biomarkers.
Methods: Adult patients with confirmed SARS-CoV-2 infection who were hospitalized from November 8, 2021, to December 31, 2021, were included. The retrospective study was conducted in Patras University Hospital, Greece. Two groups of patients were assessed, the ones who were previously vaccinated against SARS-CoV-2 (group A, n = 21), and those who were not (group B, n = 55). After analysis of peripheral blood, we calculated on admission day for each patient the total white blood cell (WBC), absolute lymphocytes count (ALC), absolute monocyte count, D-dimers, C-reactive protein (CRP) plasma levels, lactate dehydrogenase (LDH), ferritin, high-sensitive troponin, as well as the arterial oxygen partial pressure/fractional inspired oxygen (PO2/FiO2) ratio.
Results: The median age of all patients was 65.3 ± 15.2 years old; 68.4% were men and 31.6% were women. Comorbidities were present in 51 patients (67.1%). Hypertension and diabetes were observed as the most common comorbidities (33.3%). About 72.4% of the patients were unvaccinated or have received the first dose of vaccine, and 27.6% were completely vaccinated. No statistical difference was found in the total WBC count and ALC between the two groups (group A vs. group B: 8,168.95 ± 7,584.4 vs. 8,521.9 ± 6,571.3, P = 0.848 and 3,052.1 ± 7,230.7 vs. 1,279.6 ± 1,218.6, P = 0.087). Monocytes count in both groups did not show statistical difference: group A vs. group B: 672.6 ± 384.7 vs. 637.9 ± 477.8 (P = 0.754). Similarly, no difference for D-dimers (1,348.5 ± 1,397.6 vs. 1,850.9 ± 3,877.5, P = 0.575), ferritin (1,082.8 ± 1,399.5 vs. 1,327.4 ± 1,307.8, P = 0.508), high-sensitive troponin (113.6 ± 318.1 vs. 157.5 ± 48.8, P = 0.252), and CRP (6.92 ± 4.9 vs. 7.4 ± 5.9, P = 0.732). For LDH plasma levels, the statistical difference was significant (274.2 ± 85.6 vs. 387.5 ± 223.4, P = 0.003), as well as for the PO2/FiO2 ratio (355.6 ± 129.7 vs. 260.5 ± 123.3, P = 0,006).
Conclusions: In a mixed population hospitalized for COVID-19, only LDH plasma levels and the PaO2/FiO2 on admission day showed statistically significant difference between vaccinated and unvaccinated patients. Although unvaccinated patients are more likely to develop severe illness, they did not express significantly higher values of commonly used plasma biomarkers such as ferritin, CRP, and D-dimers which are related to disease severity.
Keywords: COVID-19; Vaccine; Laboratory parameters; Outcome; Alterations
| Introduction | ▴Top |
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. It took a short period to become clear that effective therapy for severe COVID-19 patients together with preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread through population vaccination is the best option worldwide. From the beginning of the pandemic, global efforts have been focused on developing safe and efficacious vaccines for COVID-19 prevention [1].
To date, COVID-19 has become increasingly a real threat due to the emergence of variants. It is so far well known that rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [2].
Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials yet [2]. In the present study, we aimed to investigate the effect of the COVID-19 vaccines on the related disease blood biomarkers on patients’ admission day.
| Materials and Methods | ▴Top |
All adult patients with confirmed SARS-CoV-2 infection who needed hospitalization in the third and fourth COVID Division of Internal Medicine Department of Patras University Hospital, Greece, from November 8, 2021, to December 31, 2021, were enrolled in the study, and remarkably, during that period when D variant genotyping was predominant. Exclusion criteria were age < 18 years and preexistent infections, such as urinary tract infections, endocarditis, and wound infections. Patients were divided into two groups, group A: patients vaccinated against SARS-CoV-2 (n = 21) and group B: patients unvaccinated against SARS-CoV-2 (n = 55). Written informed consent was obtained from all patients enrolled in the study or from a relative when necessary. The Ethics Institutional Board of the Hospital approved the study protocol. The study was conducted in compliance with the ethical standards of University Hospital of Patras, Greece on human subjects as well as with the Helsinki Declaration.
We recorded the total count of white blood cells (WBCs), absolute lymphocytes count (ALC), absolute monocyte count, D-dimers, C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, and high-sensitive troponin on admission day for each patient. Also, the arterial oxygen partial pressure/fractional inspired oxygen (PO2/FiO2) ratio upon admission was recorded. Patients’ data were extracted from the Hospital’s electronic medical records.
Statistical analysis
Statistical analysis of data was performed using SPSS-26 statistical software. Statistical values were expressed as mean ± standard deviation (SD). The minimum value of the level of statistical significance, P value, was set at 0.05. The Mann-Whitney test was used to investigate the differences of a continuous variable to two different and independent population groups.
| Results | ▴Top |
A total number of 76 adult patients (≥ 18 years old) with laboratory-confirmed SARS-CoV-2 infection via polymerase chain reaction (PCR) technique were enrolled. The median age of all patients was 65.3 ± 15.2 years old, and 68.4% were men and 31.6% were women. Comorbidities were present in 51 patients (67.1%). Hypertension and diabetes were observed as the most common comorbidities (33.3%), followed by cardiovascular disease (25.5%), atrial fibrillation (15.7%), hyperlipidemia (15.7%), and chronic obstructive pulmonary disease (13.7%). The most common symptoms on admission were fever, non-productive cough and dyspnea.
In regard to vaccination, 72.4% of the patients were unvaccinated or have received the first dose of vaccine and 27.6% were completely vaccinated. Four full vaccinated patients (19%) and 15 unvaccinated (27.3%) died during hospitalization. All critically ill patients who were admitted to intensive care unit (ICU) (n = 7, 10.5%) at the same period were unvaccinated.
When we compared the plasma levels of WBC counts, ALC, monocytes count, D-dimers, CRP, ferritin, LDH, troponin and PO2/FiO2 ratio in the two studied populations, we found the that the WBC count and ALC between the two groups did not reveal statistically significant differences (group A vs. group B: 8,168.95 ± 7,584.4 vs. 8,521.9 ± 6,571.3, (P = 0.848) and 3,052.1 ± 7,230.7 vs. 1,279.6 ± 1,218.6, (P = 0.087)). Monocytes count in both groups was as follows: group A vs. group B: 672.6 ± 384.7 vs. 637.9 ± 477.8 (P = 0.754). Analysis of the rest parameters showed: D-dimer (1,348.5 ± 1,397.6 vs. 1,850.9 ± 3,877.5, P = 0.575), ferritin (1,082.8 ± 1,399.5 vs. 1,327.4 ± 1,307.8, P = 0.508), LDH (274.2 ± 85.6 vs. 387.5 ± 223.4, P = 0.003) (Fig. 1), high-sensitive troponin (113.6 ± 318.1 vs. 157.5 ± 48.8, P = 0.252), CRP (6.92 ± 4.9 vs. 7.4 ± 5.9, P = 0.732) and PO2/FiO2 ratio (355.6 ± 129.7 vs. 260.5 ± 123.3, P = 0,006) (Fig. 1). All above values are demonstrated in Table 1. Surprisingly, the outcome did not show statistically significant difference between the two groups (group A vs. group B).
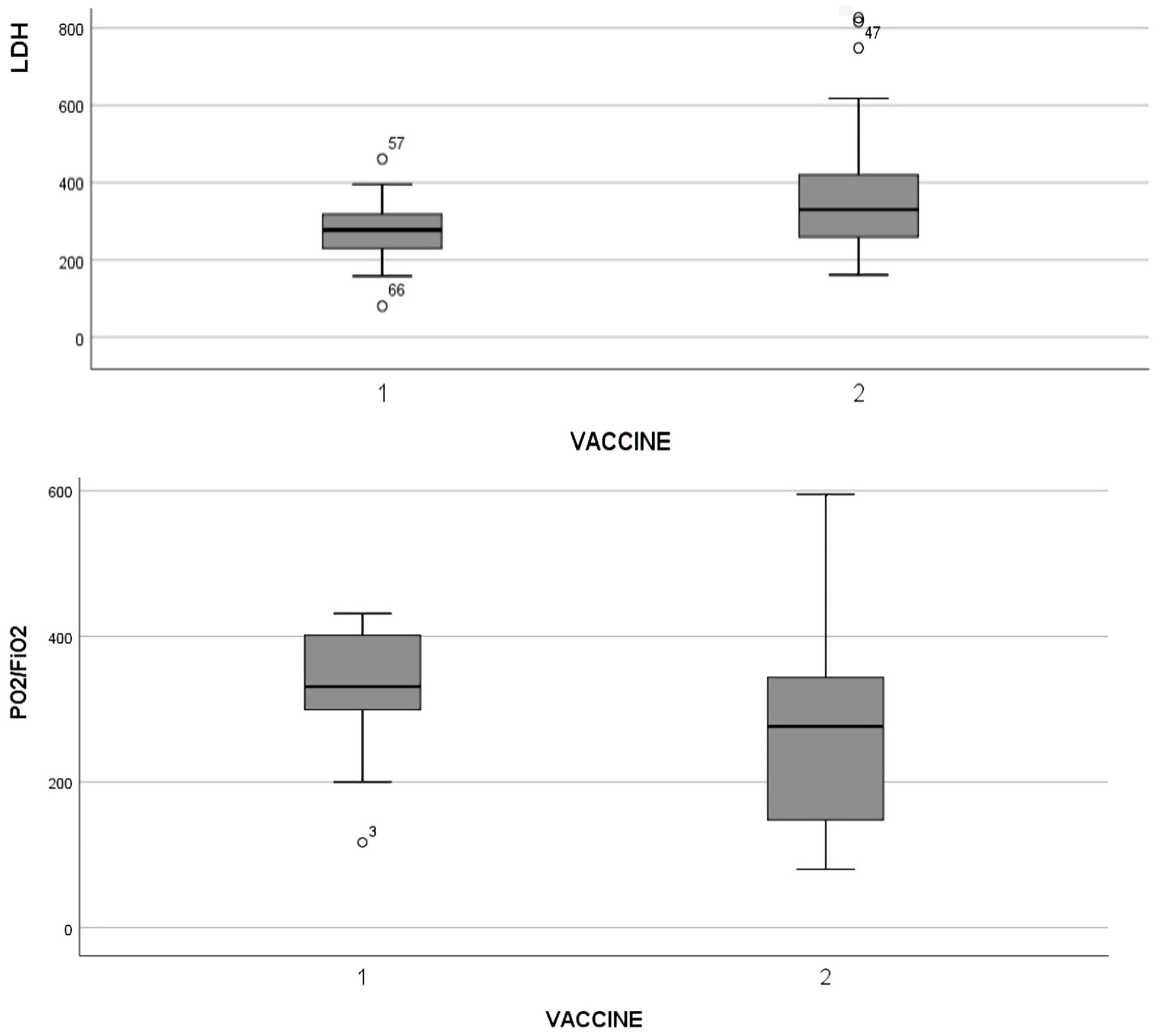 Click for large image | Figure 1. Statistically significant difference of LDH and PaO2/FiO2 ratio in adults with COVID-19. The box plots show the data from all patients analyzed. In every box plot, the upper line is the highest measurement detected. All measurements are grouped as vaccinated (1) and unvaccinated (2) patients. COVID-19: coronavirus disease 2019; LDH: lactate dehydrogenase; PO2/FiO2: arterial oxygen partial pressure/fractional inspired oxygen. |
 Click to view | Table 1. Epidemiological Data, Laboratory Findings and Outcome of the Two Groups of Patients |
| Discussion | ▴Top |
Over the last year, we have witnessed the development and clinical introduction of very effective COVID-19 vaccines, based on results from phase-3 randomized controlled trials. The two-dose regimen of BNT162b2 and mRNA-1273 mRNA, two vaccines that based on new mRNA technology, provided extremely effective protection against COVID-19 (95% and 94.1%, respectively). Different regimens of viral-vector vaccines expressing SARS-CoV-2 S protein, GamCOVID-Vac, Ad26.COV2.S and ChAdOx1, were highly effective to protect against symptomatic COVID-19 (91.6%, 66.9%, and 66.7%, respectively) [3]. Two doses of SARS-CoV-2 vaccines have been highly effective at preventing hospitalization, onset of clinical deterioration and deaths resulting from COVID-19. Moreover, SARS-CoV-2 vaccines have effectively reduced infections resulting from SARS-CoV-2 across the world, symptomatic cases, severe illness, and mortality rates [2].
Although a good knowledge has been gained on efficacy and safety of SARS-CoV-2 vaccines, which means that people who are fully vaccinated against COVID-19 have a significantly reduced risk of severe illness and need for ICU admission, less clear information has been provided on laboratory abnormalities related to disease severity in vaccinated patients when admitted for hospitalization in comparison to unvaccinated ones.
In the present retrospective study, in terms of laboratory findings, there were no statistically significant differences between vaccinated and unvaccinated individuals in the plasma levels of D-dimers, high-sensitive troponin and inflammatory markers including CRP and ferritin. Our analysis revealed statistically significant elevated LDH in the group of patients who were unvaccinated against SARS-COV-2 and were admitted to hospital. Mardani et al and Zhou et al reported that elevated LDH level on admission of SARS-CoV-2 patients, was independent predictor factor of severe disease and for an adverse clinical outcome [4, 5]. Increased plasma LDH levels has been associated also with higher risk of acute respiratory distress syndrome (ARDS), ICU support and death across published studies [6].
Some limitations of the current study should be acknowledged. First, the presented study is retrospective with a limited number of patients. Second, there is no information provided about onset of symptoms or on which day of the illness the patients are admitted to hospital care. However, all our patients were immediately admitted to hospital from the onset of symptoms. Huang et al [7] and Wang et al [8] highlighted an association between lymphocytopenia and need for ICU care, whereas Wu et al [9] showed an association between lymphocytopenia and ARDS development [6]. Although lymphocytopenia has been well described in a retrospective analysis of patients in Hong Kong and Singapore afflicted with SARS in 2003 and was associated with adverse outcomes and ICU admission, we did not find statistically significant lower ALC in the group of unvaccinated patients [10].
PaO2/FiO2 ratio is the most common used oxygenation index and was found statistically significant lower in group B (unvaccinated patients). Lung is the most organ most invaded by SARS-CoV-2. Several COVID-19 patients are characterized by hypoxia and respiratory distress. Post-mortem histological examination revealed hyaline membranes, and mixed inflammatory cell infiltration of the interstitium, alveoli, and perivascular areas, which are consistent with the characteristics of ARDS. PaO2/FiO2 has been reported to be an independent predictor of COVID-19 death [11].
In this study, we provide evidence, for the first time to our knowledge, that some very commonly used laboratory values, except LDH and PaO2/FiO2 that are used as independent predictive factors in SARS-CoV-2 infection, show no statistically significant difference between vaccinated and unvaccinated patients. Although unvaccinated patients, admitted to hospital due to SARS-CoV-2 infection, are more likely to develop severe illness, and one would expect to have significantly higher values of parameters such as ferritin, CRP, D-dimers, this has not been demonstrated in the present study.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Informed Consent
Written informed consent was obtained from all patients enrolled in the study or from a relative when necessary.
Author Contributions
ML contributed to the study design, did the literacy search and statistical analyses, and wrote the manuscript. TP, GE, DP, AC, and CP collected the data, and contributed to the manuscript writing. DV and MM gave the final approval.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11(1):22777.
doi pubmed - Liu Q, Qin C, Liu M, Liu J. Efectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infectious Diseases of Poverty. 2021;10:132.
doi pubmed - Agrawal U, Katikireddi SV, McCowan C, Mulholland RH, Azcoaga-Lorenzo A, Amele S, Fagbamigbe AF, et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2.57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir Med. 2021;9(12):1439-1449.
doi - Mardani R, Ahmadi Vasmehjani A, Zali F, Gholami A, Mousavi Nasab SD, Kaghazian H, Kaviani M, et al. Laboratory parameters in detection of COVID-19 patients with positive RT-PCR; a diagnostic accuracy study. Arch Acad Emerg Med. 2020;8(1):e43.
- Zhou Y, Zhang Z, Tian J, Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann Palliat Med. 2020;9(2):428-436.
doi pubmed - Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834-847.
doi pubmed - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi - Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
doi pubmed - Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943.
doi pubmed - Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, Mucheli SS, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131-E134.
doi - Gu Y, Wang D, Chen C, Lu W, Liu H, Lv T, Song Y, et al. PaO2/FiO2 and IL-6 are risk factors of mortality for intensive care COVID-19 patients. Sci Rep. 2021;11(1):7334.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


