| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 14, Number 11, November 2022, pages 448-457
Hypertension as One of the Main Non-Myocardial Infarction-Related Causes of Increased Cardiospecific Troponins: From Mechanisms to Significance in Current Medical Practice
Aleksey Michailovich Chaulina, b, c
aDepartment of Cardiology and Cardiovascular Surgery, Samara State Medical University, Samara 443099, Russia
bDepartment of Histology and Embryology, Samara State Medical University, Samara 443099, Russia
cEmail:
Manuscript submitted July 17, 2022, accepted August 20, 2022, published online November 29, 2022
Short title: Increased Cardiospecific Troponins and HT
doi: https://doi.org/10.14740/jocmr4796
- Abstract
- Introduction: Biochemistry and Diagnostic Contribution of Cardiospecific Troponins (CTs)
- HT as a Significant Cause of Increased CTs: Mechanisms of MC Injury and CTs Increase
- Predictive Significance of CT in Patients With HT
- Conclusions and Future Directions
- References
| Abstract | ▴Top |
It is well known that many pathological conditions of both cardiovascular diseases (CVDs) (coronary heart disease, myocardial infarction, arrhythmias, myocarditis, cardiomyopathy, etc.) and non-cardiac (sepsis, anemia, kidney diseases, diabetes mellitus, etc.) origin in the course of their development cause injury to contractile cardiac muscle cells - myocardial cells (MCs). One of the most sensitive and specific criteria for detecting MC injury are cardiospecific troponins (CTs), which are regulatory protein molecules that are released into the blood serum from MC upon their death or injury. Current methods for determining CTs are called high-sensitive ones, and their main advantage is a very low minimum detectable concentration (limit of detection) (average 1 - 10 ng/L or less), which allows early detection of minor MC injury at the earliest stages of CVDs, and therefore they can change the understanding of disease development mechanisms and open up new diagnostic possibilities. One of the most common and dangerous early diseases of the cardiovascular system is hypertension (HT). The novelty of this article lies in the discussion of a new diagnostic direction - predicting the risk of developing CVDs and their dangerous complications in patients with HT by determining the concentration of CTs. In addition, pathophysiological mechanisms underlying MC injury and the release of CTs into the bloodstream and the elimination of CTs into the urine are proposed. This information will contribute to additional fundamental and clinical research to verify the new diagnostic possibility of using CTs in clinical practice (for the management of patients with HT).
Keywords: Myocardial cells; Injury; Cardiovascular diseases; Hypertension; Cardiospecific troponins; High-sensitive; Predictive significance
| Introduction: Biochemistry and Diagnostic Contribution of Cardiospecific Troponins (CTs) | ▴Top |
The search for new diagnostic directions plays an important role in modern clinical medicine to improve the management of cardiovascular diseases (CVDs) [1, 2]. The contribution of CTs in modern diagnostics of CVDs cannot be overestimated. These laboratory biomarkers are considered the most sensitive and specific indicators of myocardial injury, which allows considering them as the “gold standard” for diagnosing acute coronary heart disease - myocardial infarction [3-6]. CTs are protein molecules, which together with another protein are called tropomyosin, form the troponin-tropomyosin system, which is an important component of thin (also called actin) filaments [6-8]. Among the proteins that are part of thin filaments, only cardiospecific troponin T (CT-T) and cardiospecific troponin I (CT-I) have a specific structure characteristic of the main cells of the heart muscular membrane - contractile myocardial cells (MCs). All other thin filament proteins, including actin, tropomyosin, and troponin C, have the same structure. CTs are significant contributors due to participating in the regulation of the contraction-relaxation of operating MC. The name of CTs is in accordance with their function. Troponin C binds to calcium ions, which enter the cytoplasm mainly from the sarcoplasmic reticulum, in which calcium channels open when a nerve impulse is transmitted. CT-T provides attachment of the troponin-tropomyosin system to thin filaments, and after troponin C binding to calcium ions, participates in the conformational movements of the troponin-tropomyosin system for the subsequent opening of myosin heads binding areas on actin. Interaction of the last two proteins with the formation of transverse actomyosin bridges underlies the contraction of striated muscles. CT-I, on the contrary, is active during muscle relaxation and blocks the formation of actomyosin bridges. Mutations in the genes encoding CTs lead to pronounced disorders of MC contraction-relaxation (hereditary cardiomyopathies), which are clinically manifested by a group of symptoms of chronic congestive cardiac failure (shortness of breath, general weakness, defatigation, edema, etc.) [9-12].
Heart contractile proteins, CT-T and CT-I are released from the cell into the blood during ischemic necrosis of MC, which can be used in the diagnosis of myocardial infarction [3-6]. The use of CTs for the diagnosis of myocardial infarction is regulated in a number of current recommendations of the European, American and Russian Cardiological Societies [6, 13-15].
Although currently the main area of CTs application in routine practices is the diagnosis of myocardial infarction, the diagnostic significance of these biomarkers is far beyond this acute CVD. This is confirmed by a number of clinical and experimental studies that reported positive levels of CTs in the blood of patients who had non-cardiac diseases (sepsis, anemia, kidney diseases, stroke, chronic obstructive pulmonary disease, diabetes mellitus, who took cardiotoxic drugs to treat the underlying cancer, and other causes) [16-24], and non-ischemic CVDs (cardiac tachyarrhythmias, cardiomyopathy, myocarditis, cardiac failure, and a number of other causes) [25-28]. Some researchers report diagnostically significant increases in CTs levels during physiological processes (for example, during physical activity [7, 29], the effect of stress on the human body [30], age- and gender-related features of CTs concentration [31, 32], as well as the effect of daily biorhythms to CTs levels) [33-36]. In general, these papers indicate the presence of many other mechanisms (not caused by ischemic necrosis) of CTs released from MC. Therefore, CTs may indicate the presence of myocardial infarction only if patients have symptoms of myocardial ischemia (chest pain typical for myocardial infarction, ischemic changes detected using electrocardiography and echocardiography). Many papers report a very high number of non-infarction related cases of increased CTs serum level. For example, according to a large retrospective study by Lindner et al, almost 90% of cases of increased CT-T were caused not by myocardial infarction, but by other diseases: acute pulmonary embolism, kidney diseases, dissecting aortic aneurysm, chronic congestive cardiac failure, inflammatory diseases of the heart membranes (myocarditis, perimyocarditis), strenuous exercise, destruction of striated muscle tissue (rhabdomyolysis), treatment of the underlying cancer using cardiotoxic chemotherapy, infiltrative disorders (e.g., amyloidosis), radiofrequency ablation therapy, defibrillator discharges, chest trauma, systemic inflammation (sepsis), shock, exacerbation of chronic obstructive pulmonary disease and diabetic ketoacidosis [28]. In this regard, it is necessary to be extremely careful in interpreting the increased CTs serum levels and take into account data obtained by using other diagnostic methods (case history, physical examination, functional diagnostics) before conducting additional potentially dangerous invasive studies (coronary angiography), final diagnosis and therapy. The main reasons for the increase in CTs levels are shown in Figure 1.
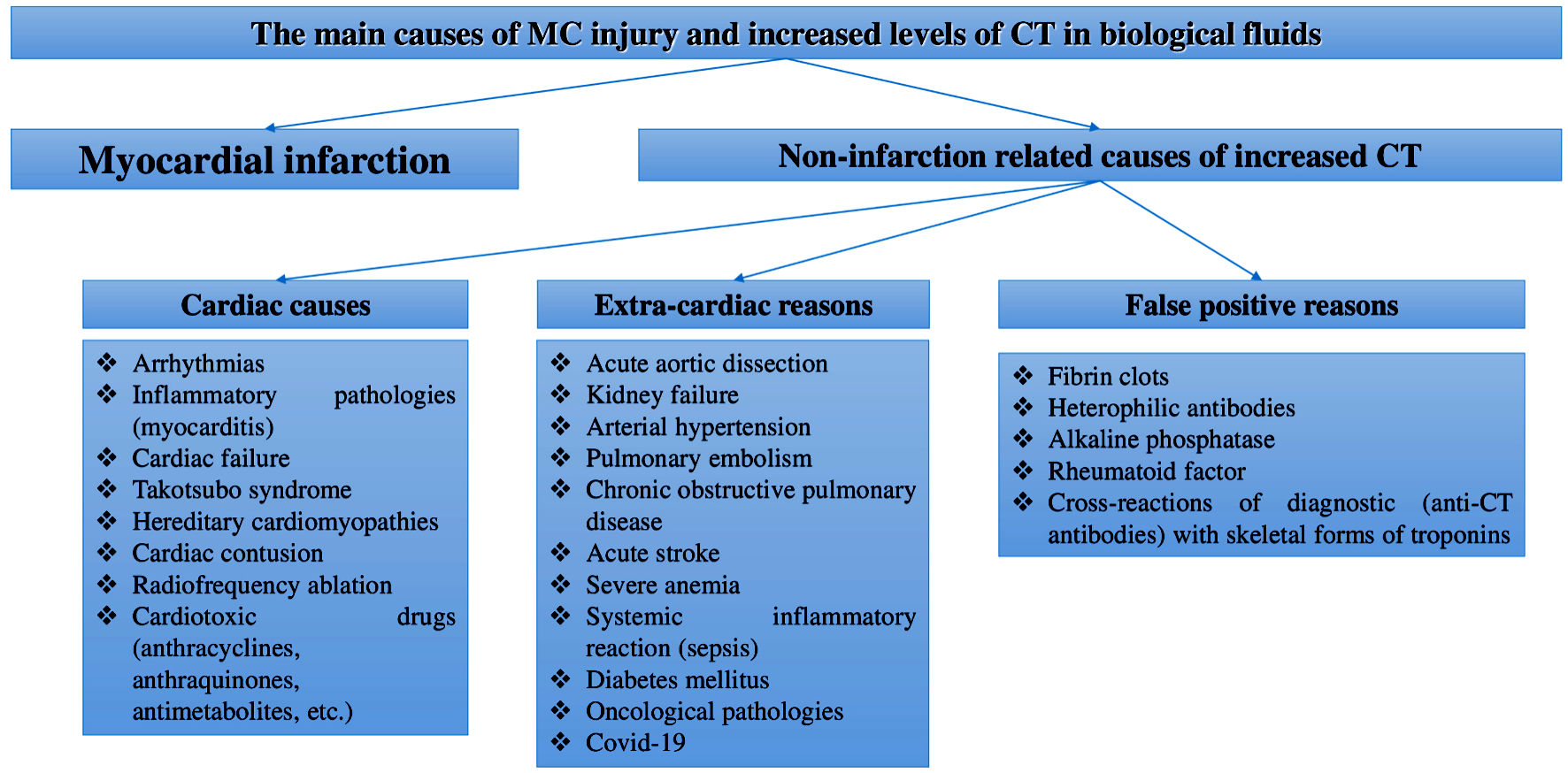 Click for large image | Figure 1. Main reasons of MC injury and increased CTs levels in body fluids. MCs: myocardial cells; CT: cardiospecific troponin; COVID-19: coronavirus disease 2019. |
With regard to the diagnostic contribution of CTs, it is necessary to note the importance of analytical characteristics of laboratory detection methods, whose improvements led to changes in a number of ideas about the biochemistry, metabolism and diagnostic significance of CTs. Current methods for determining CTs are called high-sensitive ones, and their main advantage is a very low minimum detectable concentration (limit of detection) (average 1 - 10 ng/L or less), which allows early detection of MC injury. The following features of high-sensitive methods for determining CTs are of clinical significance: 1) Early diagnosis of myocardial infarction (within 1 - 2 h from the time the pain syndrome develops or from admission to the emergency department), whereas earlier, when using moderate-sensitive methods for determining CTs, diagnosis took an average of 12 - 24 h [37]; 2) The role of gender, circadian and age factors, which, according to a number of studies, can affect the accuracy of diagnosis [31-36]; 3) The possibility to determine CTs in healthy people with risk of CVDs or those with subclinical forms of CVDs (for example, coronary heart disease, hypertension (HT), transient ischemic attacks, etc.), and increased levels of CTs in cases of these diseases has a high predictive significance in terms of identifying an unfavorable prognosis [38-41]; 4) Diagnostic significance of CTs concentrations in urine and oral fluid (saliva) in case of CVDs [42-47]. This new field of noninvasive diagnostics and monitoring of CVDs has promising prospects due to a number of advantages associated with obtaining biomaterial.
One of the most common clinical forms of CVDs is HT. This disease is also considered as a risk factor for the development of acute CVD and cerebrovascular diseases. Taking into account the high prevalence of HT and its contribution in the development and progression of dangerous cardiovascular complications, consideration of MC injury mechanisms and predictive significance of the main injury biomarkers (CTs) in case of this disease is of fundamental and practical importance, and therefore is especially noteworthy.
The novelty of this article lies in the discussion of a new diagnostic direction - predicting the risk of developing CVDs and their dangerous complications in patients with HT by determining the concentration of CTs. In addition, pathophysiological mechanisms underlying MC injury and the release of CVD into the bloodstream and the elimination of CTs into the urine are proposed. This information will contribute to additional fundamental and clinical research to verify the new diagnostic possibility of using CTs in clinical practice (for the management of patients with HT).
| HT as a Significant Cause of Increased CTs: Mechanisms of MC Injury and CTs Increase | ▴Top |
HT is one of the leading risk factors for the development of acute CVDs, which result in the development of approximately half of the cases of acute coronary heart disease and acute ischemic strokes [48]. An additional negative factor of HT is the perfidy of this disease, which is in the lack of symptoms in the early stages of its development and the gradual adaptation of patients to high blood pressure (BP), so many cases of HT remain underdiagnosed. As a result, the first clinically significant manifestations of HT, which force patients to go to emergency departments, are acute cardiovascular complications, such as myocardial infarction, transient ischemic attacks, strokes, etc. Thus, Caligiuri et al detected high BP in 50% of workers of a number of urban enterprises who had never previously complained of BP and therefore did not take drugs to normalize it. In addition, systolic and diastolic pressures exceeded 180 and 120 mm Hg in 2% of the tested persons - this values correspond to a hypertensive crisis and require emergency treatment [48].
According to the current classification, HT is divided into two main types, primary (essential) HT and secondary (symptomatic) HT [49, 50]. Essential HT is much more common (90-95% of all cases of HT); secondary HT and the exact causes of its development are still unknown. Secondary HT is diagnosed in about 5-10% of individuals with high BP. The causes of secondary HT are pathologies of other organs and systems (endocrine, urinary, nervous, etc.). In other words, secondary HT develops during the progression of the primary disease, which, as a rule, is accompanied by violations of the regulation of the volume of circulating blood and/or vascular tone. The most common types of secondary HT are: endocrine (primary aldosteronism, Cushing’s syndrome, acromegaly, thyrotoxicosis, acromegaly, etc.), renal (renal parenchymal disease, renin-producing tumor, etc.), reno-vascular (atherosclerotic), iatrogenic (administration of drugs that increase BP) and gestational (pregnancy-induced HT) and others [49, 50].
According to a number of recent studies, HT can be considered as a significant cause of increased CTs in both the blood serum [51-53] and urine of patients [54-56]; however, the pathophysiological mechanisms that cause an increase in CTs levels and MC injury have not been fully established. Taking into account the mechanisms of HT development, the following key pathophysiological mechanisms can be identified that underlie MC injury and CTs release into the bloodstream: 1) Myocardial hypertrophy due to increased load on the myocardium; 2) Increased apoptosis due to hyperactivity of the sympathoadrenal system or increased load on the myocardium; 3) Injury to MC membranes, which leads to an increase in membrane permeability and CTs release; 4) The effect of BP on the glomerular filtration rate, which is important in the elimination of CTs from the bloodstream.
Myocardial hypertrophy
High BP causes an increase in preload on the myocardium, which causes its compensatory restructuring and the formation of hypertrophy, which is manifested by an increase in MC volume. With an increase in MC volume, the level of CTs release into the bloodstream increases as a result of metabolism and renewal of MC [57-59]. This is evidenced by clinical studies that have revealed connection of myocardial hypertrophy with CTs levels. An additional indirect reasoning of this mechanism is also the research that revealed the gender characteristics of CTs levels [60, 61]. At the same time, the main mechanism for the formation of gender characteristics, according to the authors, is the mass of the myocardium, which is higher in men than in women [61, 62]. Similar features are also characteristic of the metabolism of striated skeletal muscles, which is manifested by gender differences in the levels of creatine phosphokinase and its MB isoform, skeletal troponins, creatinine, and other muscle tissue metabolites [63]. However, the most important proof of this mechanism functioning, i.e., the fact that more CTs molecules are released from hypertrophic MC are clinical studies that have revealed a correlation of CTs levels with myocardial hypertrophy in healthy individuals and patients with HT [64, 65].
Apoptosis of MC
Apoptosis of cardiac muscle tissue cells in case of HT develops as a result of myocardium walls stretching, increased preload, and hyperactivation of sympathoadrenal system, which has been shown in a number of experimental and clinical studies [66-69]. Thus, Cheng et al, when studying the effect of myocardium stretch on the processes of MC apoptosis, noted that in MC the generation of reactive oxygen species (2.4 times) and Fas protein expression (21 times) increase from the stretch zone, which indicates a significant increase in apoptosis in response to myocardium stretch [66]. Weil et al, by increasing the preload on MC and increasing BP by administering phenylephrine to laboratory pigs, also noted a significant activation of programmed death of MC compared with animals that had placebo (31.3 ± 11.9 vs. 4.6 ± 3.0 MC in state of apoptosis/cm2; P < 0.01). In addition, a high degree of MC apoptosis in the experimental group was accompanied by a sharp increase in CT-I levels (856 ± 956 ng/L after 1 h and 1,462 ± 1,691 ng/L after 24 h) [67]. Finally, hyperactivation of the sympathoadrenal system can be noted as another important factor that enhances MC apoptosis in HT. Studies on cardiac myocytes in vitro showed that beta-agonists enhance MC apoptosis through cAMP-dependent activation of voltage-operated calcium channels and overload of MC with calcium ions [68] and NF2-signaling pathway leading to c-Jun activation of N-terminal kinases, increased levels of cytosolic cytochrome c and Bax expression [69].
Injury to MC cell membranes and increased permeability
State of the cell membrane is one of the key factors affecting the degree of cytosolic protein molecules released into the bloodstream from the intracytoplasmic space. Many pathological conditions are accompanied by the release of cytoplasmic biomarkers into the bloodstream even before cell death (irreversible injury). For example, the release of liver enzymes (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase) and muscle markers (creatine phosphokinase and its isoforms, skeletal troponin isoforms, myoglobin) in the early stages of inflammatory diseases of the hepatobiliary tract and skeletal myopathies, respectively, when there are no symptoms of necrosis yet [70-72]. CTs are localized both within the troponin system on thin filaments (structural or non-free CTs fraction) and freely (cytoplasmic or free CTs fraction). There is some evidence that approximately 95% of the total CTs content in MC is in the composition of the structural fraction, and about 5% is in the composition of the cytoplasmic fraction (Fig. 2).
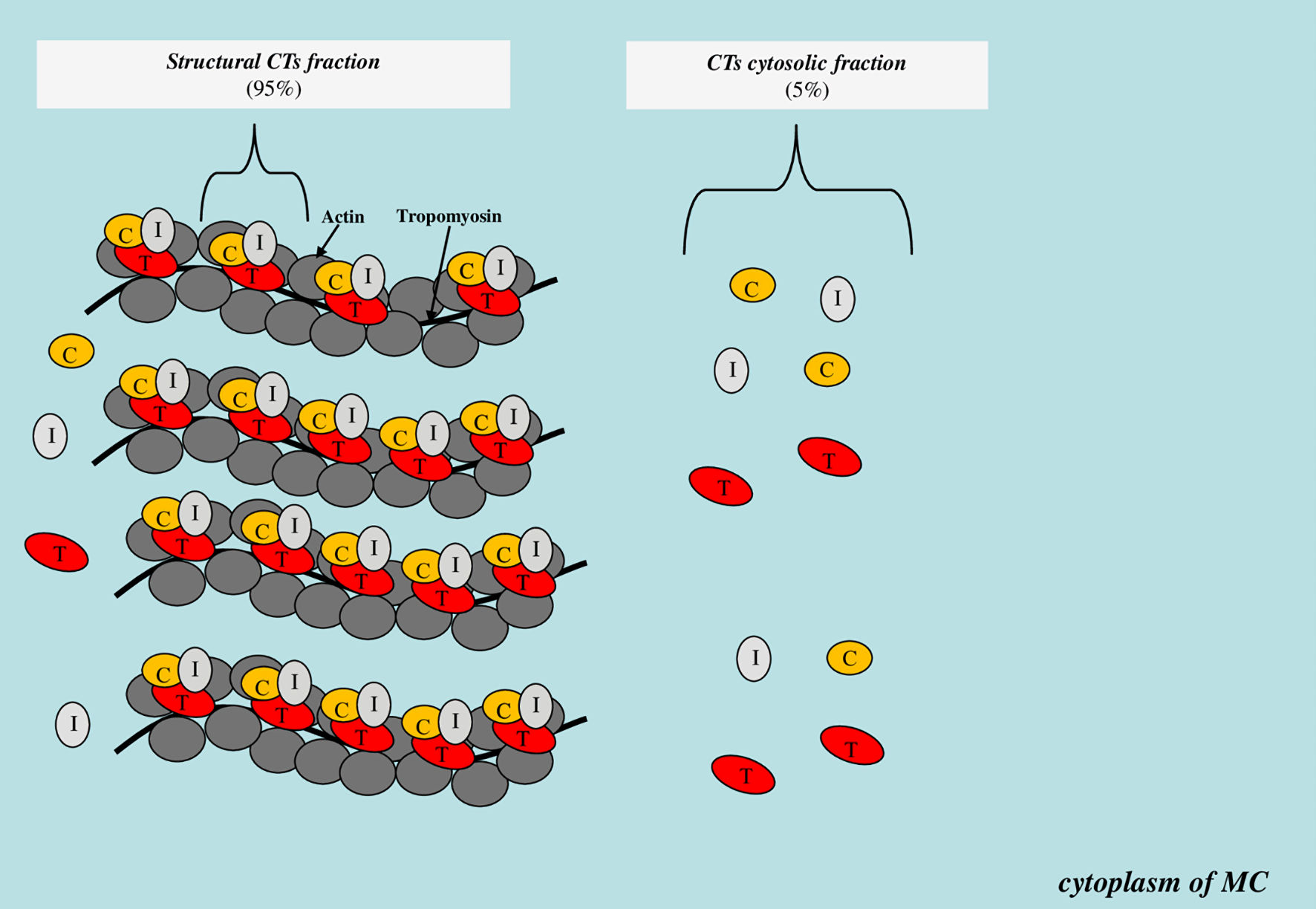 Click for large image | Figure 2. Scheme of localization of the structural and cytoplasmic CTs fraction. CTs: cardiospecific troponins; C: troponin C; T: troponin T; I: troponin I; MC: myocardial cell. |
A number of authors believe that CTs cytosolic fraction can exit the MC cytoplasm into the bloodstream with minor and reversible MC injury that occurs during physical activity [29] or under the influence of psychoemotional stress [30]. The degree of increase in CTs concentrations in case of such injury is small, amounting to only 3 - 5 times from the upper reference limit, which is due to the relatively small volume of freely localized CTs molecules in the cytoplasm. In addition, the CTs cytoplasmic fraction of CT is of significant importance in the formation of circadian rhythms in both healthy individuals and patients with CVDs. The highest levels of CTs are observed in the morning, which is associated with increased activity of sympathoadrenal system [73-76], hemostasis system [77-80] and secretory activity of the thyroid gland [81-83]. According to Hessel et al, the CTs cytoplasmic fraction can be released with an increase in BP and myocardial preload. The main mechanism responsible for this increase in CTs levels is MC transmembrane receptor proteins, in particular integrins. Stimulation of the latter is accompanied by the activation of enzymes (matrix metalloproteinases and calpain), which cause injury (increase in permeability) of MC membrane and CTs release into the bloodstream [84]. In addition to increase in permeability of MC membrane, these enzymes can also promote CTs fragmentation into small fragments that can freely pass through the cell membrane [85, 86].
Effect of BP on glomerular filtration rate and elimination of CTs from the bloodstream
The most important factors influencing the CTs levels in the blood serum are the mechanisms of their elimination from the bloodstream. At the same time, the urinary system is of significant importance in the elimination of CTs. A number of studies have reported a high prevalence of increased CTs levels in patients with urinary system diseases, in particular kidney failure, which, therefore, should be considered as a significant and non-infarction related cause of increased CTs [16, 87, 88]. A correlation has been noted between the degree of kidney failure, determined on the basis of glomerular filtration rate, and the degree of CTs increase in patients without symptoms of CVD [16]. Factors that increase the glomerular filtration rate, in particular high BP, on the contrary, increase the elimination of CTs from the bloodstream into the urine, as shown in the study by Pervan et al [54]. This fact is of great practical importance, which is in the possibility of using urine as a biomaterial for monitoring the course of HT and assessing its prognosis. Another factor affecting the glomerular filtration of proteins is the state of the kidney filter. Injury to the latter in glomerulopathies often leads to increased protein excretion, and in particular CTs.
One of the possible explanations for how CTs pass through the glomerular filter is the proteolytic cleavage of CTs under the influence of a number of intra- and extracellular proteinases. Thus, the size of a protein molecule is associated with the possibility of its passage through the small pores of the glomerular filter. Low-molecular-weight proteins, in contrast to high-molecular-weight proteins, are usually found in small amounts in primary urine, indicating a relationship between filtration and molecular size [86-90]. CTs under the influence of a number of intracellular and extracellular proteolytic enzymes are fragmented into several dozen fragments, the molecular weight of which is extremely small, which probably allows them to be more actively eliminated from the bloodstream. CTs elimination is possible not only through the glomerular filter, but also through other barriers, in particular, hematosalivary, blood-brain into saliva and cerebrospinal fluid, respectively, which is confirmed by relevant studies [44-49, 54, 90-92]. However, the processes of proteolytic cleavage of troponins inside cells and in blood serum have been studied very little. Although researchers have reported dozens of fragments of various molecular weights and sizes, all of the enzymes that are responsible for cleavage of troponins and the formation of such a significant number of fragments are not known. There are extremely few studies that are focused on the specific mechanisms of proteolytic cleavage of CTs. One such studies conducted by Russian and Finnish researchers led by Katrukha [93] reported that the enzyme thrombin catalyzes the specific cleavage of CT-T into two fragments. It is noteworthy that under conditions of high BP in patients, activation of this enzyme is observed [94], and accordingly, the processes of proteolytic cleavage of troponins into small fragments are intensified and the glomerular filtration rate is increased, which contributes to the elimination of formed small fragments of CTs through the glomerular filter from the bloodstream into the urine. The identification of all CTs that affect proteolytic cleavage is important for understanding this process and improving laboratory diagnostics, including the use of urine as a noninvasive biomaterial. Among the most valuable cardiospecific markers recommended for diagnosing myocardial infarction and cardiac failure, the determination of CTs and natriuretic peptides in urine has shown promising results. Recent studies by several research groups have shown the high diagnostic significance of CTs and natriuretic peptides in noninvasively obtained body fluids in individuals with coronary heart disease [46, 95], myocardial infarction [43-45, 47, 96, 97], diabetes mellitus [45], cardiac failure [98, 99] and HT [54]. Further research should be aimed at clarifying these promising diagnostic possibilities.
Summarizing the above, it is possible to propose the following pathophysiological mechanisms of MC injury and increased CTs levels in patients with HT (Fig. 3).
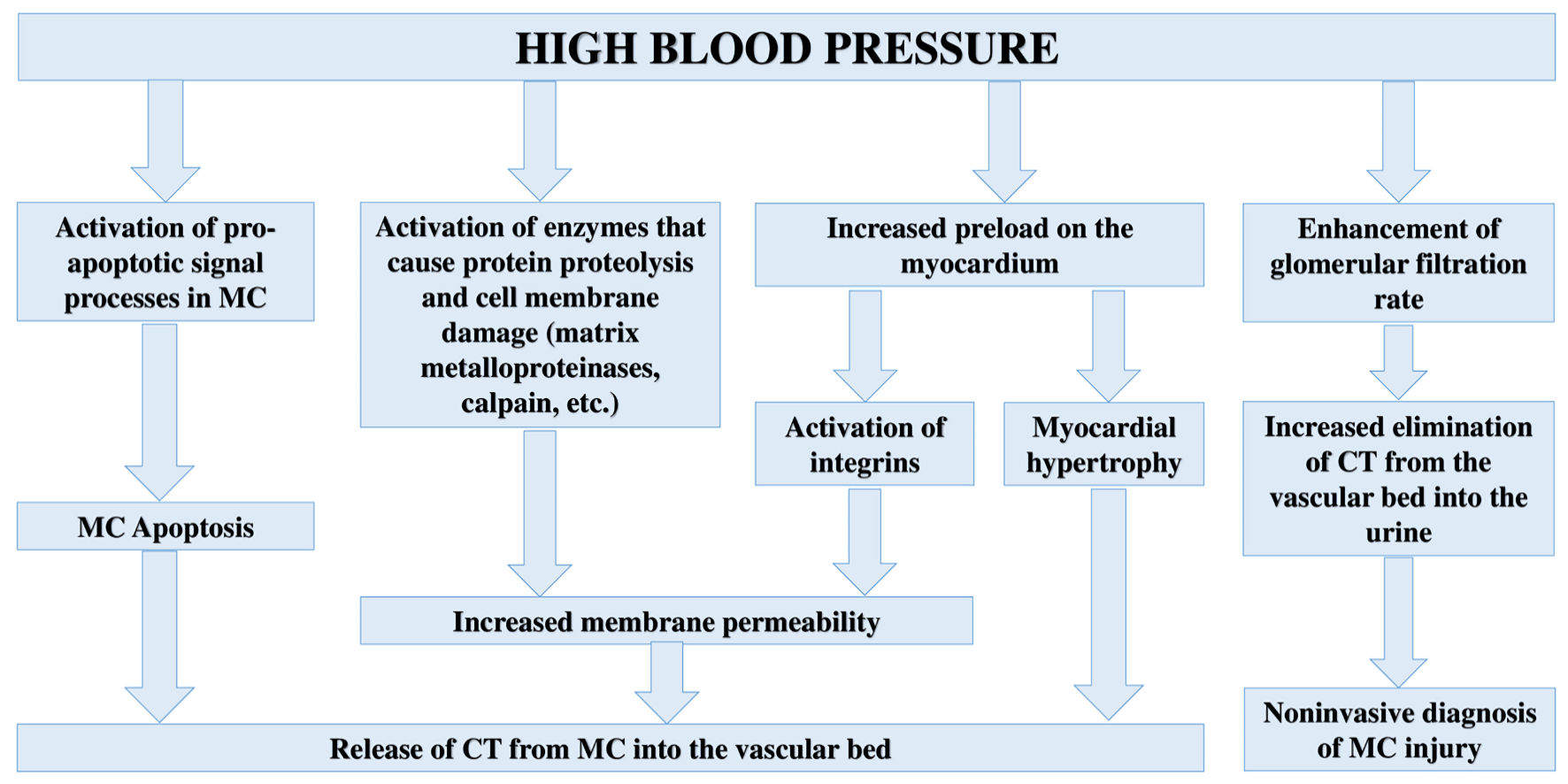 Click for large image | Figure 3. Pathophysiological mechanisms of MC injury and increased CTs levels in body fluids in case of HT. CT: cardiospecific troponin; MC: myocardial cell. |
| Predictive Significance of CT in Patients With HT | ▴Top |
The mechanisms of CTs release into the bloodstream from MC are of great practical importance, since high levels of CTs indicate injury to MC and an unfavorable prognosis for patients [52-54, 100-103]. Data on the prevalence and predictive significance of CTs in case of HT based on the results of clinical trials are presented in Table 1 [52, 54, 100-103].
 Click to view | Table 1. Clinical Studies on the Diagnostic Significance of CTs in Case of HT |
One of the important problems of troponin immunoassays is the lack of their standardization. So, there are a large number of manufacturers of methods (each of which has different analytical characteristics and reference values (99 percentile)) determination of CTs; therefore, it is impossible to establish a common reference concentration range for all troponin immunotests. Taking into account the fact that elevated levels of CTs (> 99 percentile) in patients with HT are associated with an unfavorable prognosis [52-54, 100-103], when managing patients with HT, one should rely on the standard 99 percentile provided by the manufacturer of the immunotest. However, with further study, the optimal (reference) values of CTs may change.
| Conclusions and Future Directions | ▴Top |
Based on the results of this descriptive review of the literature, it should be noted that HT is one of the most common and unrelated causes of MC injury and increased concentration of CTs. This property has an important clinical significance in modern clinical medicine (for the management of patients with HT). Thus, based on the data of recent clinical studies, it has been shown that elevated serum levels of CTs in patients with HT are associated with left ventricular hypertrophy, the risk of developing chronic congestive cardiac failure, coronary heart disease, acute CVD, as well as their complications. This new diagnostic line of research will allow us to replenish the arsenal of methods for managing patients with HT. Understanding the pathophysiological mechanisms of MC injury plays an important role in substantiating the diagnostic value of CTs. Based on the analysis of recent experimental and clinical work, I can note the following pathophysiological mechanisms of injury to MC and an increase in the concentration of CTs: 1) Death of MC as a result of activation of pro-apoptotic signaling pathways; 2) Activation of proteolytic enzymes inside MC, which causes damage to cell membranes and increased permeability to intracellular proteins, including CTs; 3) Myocardial hypertrophy (increased renewal and metabolism of MCs); 4) An increase in the first stage of urine production (glomerular filtration) and an increase in the elimination of CTs from the blood into the primary urine. Further large-scale studies should be aimed at verifying this new diagnostic direction (the prognostic value of CTs) and clarifying the pathophysiological mechanisms of MC injury in HT.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Author declares that there is no conflict of interest.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Doughty KN, Del Pilar NX, Audette A, Katz DL. Lifestyle medicine and the management of cardiovascular disease. Curr Cardiol Rep. 2017;19(11):116.
doi pubmed - Chaulin AM, Duplyakov DV. Environmental factors and cardiovascular diseases. Hygiene and Sanitation. 2021;100(3):223-228. (In Russ).
doi - Chauin A. The main causes and mechanisms of increase in cardiac troponin concentrations other than acute myocardial infarction (Part 1): Physical exertion, inflammatory heart disease, pulmonary embolism, renal failure, sepsis. Vasc Health Risk Manag. 2021;17:601-617.
doi pubmed - Chaulin A, Duplyakov D. Analytical review of modern information on the physiological and pathochemical mechanisms of the release of cardiospecific proteins from muscle tissue, methodology and technologies of their research, interpretation of the results. Laboratory Diagnostics. Eastern Europe. 2022;11(1):78-97.
doi - Santalo M, Martin A, Velilla J, Povar J, Temboury F, Balaguer J, Munoz M, et al. Using high-sensitivity troponin T: the importance of the proper gold standard. Am J Med. 2013;126(8):709-717.
doi pubmed - Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367.
doi pubmed - Chaulin A. Cardiac troponins: contemporary biological data and new methods of determination. Vasc Health Risk Manag. 2021;17:299-316.
doi pubmed - Marston S, Zamora JE. Troponin structure and function: a view of recent progress. J Muscle Res Cell Motil. 2020;41(1):71-89.
doi pubmed - Chaulin AM. False-positive causes in serum cardiac troponin levels. J Clin Med Res. 2022;14(2):80-87.
doi pubmed - Parvatiyar MS, Pinto JR, Dweck D, Potter JD. Cardiac troponin mutations and restrictive cardiomyopathy. J Biomed Biotechnol. 2010;2010:350706.
doi pubmed - Mogensen J, Hey T, Lambrecht S. A systematic review of phenotypic features associated with cardiac troponin i mutations in hereditary cardiomyopathies. Can J Cardiol. 2015;31(11):1377-1385.
doi pubmed - Tobacman LS, Cammarato A. Cardiomyopathic troponin mutations predominantly occur at its interface with actin and tropomyosin. J Gen Physiol. 2021;153(3):e202012815.
doi pubmed - Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618-e651.
doi pubmed - Russian Society of Cardiology. Clinical practice guidelines for acute ST-segment elevation myocardial infarction. Russian Journal of Cardiology. 2020;25(11):4103. (In Russ).
doi - Barbarash OL, Duplyakov DV, Zateischikov DA, Panchenko EP, Shakhnovich RM, Yavelov IS, et al. 2020 Clinical practice guidelines for acute coronary syndrome without ST segment elevation. Russian Journal of Cardiology. 2021;26(4):4449. (in Russian).
doi - Dubin RF, Li Y, He J, Jaar BG, Kallem R, Lash JP, Makos G, et al. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013;14:229.
doi pubmed - Chaulin AM, Duplyakov DV. Comorbidity in chronic obstructive pulmonary disease and cardiovascular disease. Cardiovascular Therapy and Prevention. 2021;20(3):2539. (in Russian).
doi - Nolte CH, Scheitz JF, Endres M. [Troponin elevation in ischemic stroke patients]. Med Klin Intensivmed Notfmed. 2017;112(3):222-226.
doi pubmed - Ananthan K, Lyon AR. The role of biomarkers in cardio-oncology. J Cardiovasc Transl Res. 2020;13(3):431-450.
doi pubmed - Chaulin AM, Duplyakov DV. Arrhythmogenic effects of doxorubicin. Complex Issues of Cardiovascular Diseases. 2020;9(3):69-80. (In Russ).
doi - Chaulin AM, Duplyakov DV. Cardioprotective strategies for doxorubicin-induced cardiotoxicity: present and future. Rational Pharmacotherapy in Cardiology. 2022;18(1):103-112. (in Russian).
doi - Rezende PC, Everett BM, Brooks MM, Vlachos H, Orchard TJ, Frye RL, Bhatt DL, et al. Hypoglycemia and Elevated Troponin in Patients With Diabetes and Coronary Artery Disease. J Am Coll Cardiol. 2018;72(15):1778-1786.
doi pubmed - Chaulin AM. Elevation mechanisms and diagnostic consideration of cardiac troponins under conditions not associated with myocardial infarction. Part 2. Life (Basel). 2021;11(11):1175.
doi pubmed - Eggers KM, Lindahl B. Application of cardiac troponin in cardiovascular diseases other than acute coronary syndrome. Clin Chem. 2017;63(1):223-235.
doi pubmed - Chaulin AM. Cardiac troponins metabolism: from biochemical mechanisms to clinical practice (literature review). Int J Mol Sci. 2021;22(20):10928.
doi pubmed - Stavroulakis GA, George KP. Exercise-induced release of troponin. Clin Cardiol. 2020;43(8):872-881.
doi pubmed - Chaulin AM. Elevation mechanisms and diagnostic consideration of cardiac troponins under conditions not associated with myocardial infarction. Part 1. Life (Basel). 2021;11(9):914.
doi pubmed - Lindner G, Pfortmueller CA, Braun CT, Exadaktylos AK. Non-acute myocardial infarction-related causes of elevated high-sensitive troponin T in the emergency room: a cross-sectional analysis. Intern Emerg Med. 2014;9(3):335-339.
doi pubmed - Chaulin AM, Dupliakov DV. Physical activity and cardiac markers: part 1. Human Sport Medicine. 2022;22(2):15-28.
doi - Lazzarino AI, Hamer M, Gaze D, Collinson P, Steptoe A. The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults. J Am Coll Cardiol. 2013;62(18):1694-1701.
doi pubmed - Chaulin AM. Cardiac troponins: current information on the main analytical characteristics of determination methods and new diagnostic possibilities. Medwave. 2021;21(11):e8498.
doi pubmed - Rocco E, La Rosa G, Liuzzo G, Biasucci LM. High-sensitivity cardiac troponin assays and acute coronary syndrome: a matter of sex? J Cardiovasc Med (Hagerstown). 2019;20(8):504-509.
doi pubmed - Chaulin AM, Duplyakova PD, Duplyakov DV. Circadian rhythms of cardiac troponins: mechanisms and clinical significance. Russian Journal of Cardiology. 2020;25(3S):4061. (in Russian).
doi - van der Linden N, Hilderink JM, Cornelis T, Kimenai DM, Klinkenberg LJJ, van Doorn WP, Litjens EJR, et al. Twenty-four-hour biological variation profiles of cardiac troponin I in individuals with or without chronic kidney disease. Clin Chem. 2017;63(10):1655-1656.
doi pubmed - Zaninotto M, Padoan A, Mion MM, Marinova M, Plebani M. Short-term biological variation and diurnal rhythm of cardiac troponin I (Access hs-TnI) in healthy subjects. Clin Chim Acta. 2020;504:163-167.
doi pubmed - Chaulin AM, Duplyakov DV. High-sensitivity cardiac troponins: circadian rhythms. Cardiovascular Therapy and Prevention. 2021;20(1):2639. (In Russ).
doi - Chaulin AM. Main analytical characteristics of laboratory methods for the determination of cardiac troponins: a review from the historical and modern points of view. Orv Hetil. 2022;163(1):12-20.
doi pubmed - Lyngbakken MN, Vigen T, Ihle-Hansen H, Brynildsen J, Berge T, Ronning OM, Tveit A, et al. Cardiac troponin I measured with a very high sensitivity assay predicts subclinical carotid atherosclerosis: the Akershus Cardiac Examination 1950 Study. Clin Biochem. 2021;93:59-65.
doi pubmed - Witkowski M, Wu Y, Hazen SL, Tang WHW. Prognostic value of subclinical myocardial necrosis using high-sensitivity cardiac troponin T in patients with prediabetes. Cardiovasc Diabetol. 2021;20(1):171.
doi pubmed - Laufer EM, Hofstra L. Subclinical thrombotic events as a mechanism for troponin release? J Am Coll Cardiol. 2018;71(9):1056.
doi pubmed - Hayama H, Ide S, Moroi M, Kitami Y, Bekki N, Kubota S, Uemura Y, et al. Elevated high-sensitivity troponin is associated with subclinical cardiac dysfunction in patients recovered from coronavirus disease 2019. Glob Health Med. 2021;3(2):95-101.
doi pubmed - Aydin S, Aydin S, Kobat MA, Kalayci M, Eren MN, Yilmaz M, Kuloglu T, et al. Decreased saliva/serum irisin concentrations in the acute myocardial infarction promising for being a new candidate biomarker for diagnosis of this pathology. Peptides. 2014;56:141-145.
doi pubmed - Mirzaii-Dizgah I, Riahi E. Salivary troponin I as an indicator of myocardial infarction. Indian J Med Res. 2013;138(6):861-865.
- Chaulin AM, Karslyan LS, Bazyuk EV, Nurbaltaeva DA, Duplyakov DV. [Clinical and diagnostic value of cardiac markers in human biological fluids]. Kardiologiia. 2019;59(11):66-75.
doi pubmed - Chen JY, Lee SY, Li YH, Lin CY, Shieh MD, Ciou DS. Urine high-sensitivity troponin I predict incident cardiovascular events in patients with diabetes mellitus. J Clin Med. 2020;9(12):3917.
doi pubmed - Klichowska-Palonka M, Zaleska-Chrominska K, Bachanek T. [Possibility of using saliva as a diagnostic test material in cardiovascular diseases]. Wiad Lek. 2015;68(3 pt 2):354-357.
- Chaulin AM, Duplyakova PD, Bikbaeva GR, Tukhbatova AA, Grigorieva EV, Duplyakov DV. Concentration of high-sensitivity cardiac troponin I in the oral fluid in patients with acute myocardial infarction: a pilot study. Russian Journal of Cardiology. 2020;25(12):3814. (in Russian).
doi - Caligiuri SP, Austria JA, Pierce GN. Alarming prevalence of emergency hypertension levels in the general public identified by a hypertension awareness campaign. Am J Hypertens. 2017;30(3):236-239.
doi pubmed - Rossi GP, Bisogni V, Rossitto G, Maiolino G, Cesari M, Zhu R, Seccia TM. Practice recommendations for diagnosis and treatment of the most common forms of secondary hypertension. High Blood Press Cardiovasc Prev. 2020;27(6):547-560.
doi pubmed - Tziomalos K. Secondary hypertension: novel insights. Curr Hypertens Rev. 2020;16(1):11.
doi pubmed - Harvell B, Henrie N, Ernst AA, Weiss SJ, Oglesbee S, Sarangarm D, Hernandez L. The meaning of elevated troponin I levels: not always acute coronary syndromes. Am J Emerg Med. 2016;34(2):145-148.
doi pubmed - Ucar H, Gur M, Kivrak A, Koyunsever NY, Seker T, Akilli RE, Turkoglu C, et al. High-sensitivity cardiac troponin T levels in newly diagnosed hypertensive patients with different left ventricle geometry. Blood Press. 2014;23(4):240-247.
doi pubmed - Afonso L, Bandaru H, Rathod A, Badheka A, Ali Kizilbash M, Zmily H, Jacobsen G, et al. Prevalence, determinants, and clinical significance of cardiac troponin-I elevation in individuals admitted for a hypertensive emergency. J Clin Hypertens (Greenwich). 2011;13(8):551-556.
doi pubmed - Pervan P, Svagusa T, Prkacin I, Savuk A, Bakos M, Perkov S. Urine high sensitive Troponin I measuring in patients with hypertension. Signa Vitae. 2017;13:62-64.
doi - Chaulin AM. Diagnostic value of highly sensitive cardiac troponins and mechanisms of their increase in serum and urine in arterial hypertension. La Rivista Italiana della Medicina di Laboratorio. 2021;17(2):99-107.
doi pubmed - Potkonjak AM, Sabolovic Rudman S, Nikolac Gabaj N, Kuna K, Kosec V, Stanec Z, Zovak M, et al. Urinary troponin concentration as a marker of cardiac damage in pregnancies complicated with preeclampsia. Med Hypotheses. 2020;144:110252.
doi pubmed - Graham E, Bergmann O. Dating the heart: exploring cardiomyocyte renewal in humans. Physiology (Bethesda). 2017;32(1):33-41.
doi pubmed - Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98-102.
doi pubmed - White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57(24):2406-2408.
doi pubmed - Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63(14):1441-1448.
doi pubmed - Giannitsis E, Mueller-Hennessen M, Zeller T, Schuebler A, Aurich M, Biener M, Vafaie M, et al. Gender-specific reference values for high-sensitivity cardiac troponin T and I in well-phenotyped healthy individuals and validity of high-sensitivity assay designation. Clin Biochem. 2020;78:18-24.
doi pubmed - Chaulin AM, Abashina OE, Duplyakov DV. High-sensitivity cardiac troponins: detection and central analytical characteristics. Cardiovascular Therapy and Prevention. 2021;20(2):2590. (in Russian).
doi - Neal RC, Ferdinand KC, Ycas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med. 2009;122(1):73-78.
doi pubmed - McEvoy JW, Chen Y, Nambi V, Ballantyne CM, Sharrett AR, Appel LJ, Post WS, et al. High-sensitivity cardiac troponin T and risk of hypertension. Circulation. 2015;132(9):825-833.
doi pubmed - Stabouli S, Kotsis V, Rizos Z, Toumanidis S, Karagianni C, Constantopoulos A, Zakopoulos N. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. 2009;24(8):1545-1551.
doi pubmed - Cheng W, Li B, Kajstura J, Li P, Wolin MS, Sonnenblick EH, Hintze TH, et al. Stretch-induced programmed myocyte cell death. J Clin Invest. 1995;96(5):2247-2259.
doi pubmed - Weil BR, Suzuki G, Young RF, Iyer V, Canty JM, Jr. Troponin release and reversible left ventricular dysfunction after transient pressure overload. J Am Coll Cardiol. 2018;71(25):2906-2916.
doi pubmed - Dalal S, Connelly B, Singh M, Singh K. NF2 signaling pathway plays a pro-apoptotic role in beta-adrenergic receptor stimulated cardiac myocyte apoptosis. PLoS One. 2018;13(4):e0196626.
doi pubmed - Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189(3):257-265.
doi pubmed - Nikonorova MA, Karbysheva NV, Kozhevnikova GM, Mamaev AN, Shevchenko VV, Beskhlebova OV. Parenteral viral hepatitis in people with hemophilia: ways to solve the problem. Bulletin of Medical Science. 2019;1(13):52-56.
doi - Chaulin AM, Abashina OE, Duplyakov DV. Pathophysiological mechanisms of cardiotoxicity in chemotherapeutic agents. Russian Open Medical Journal. 2020;9:e0305.
doi - Evdokimova NE, Tsygankova OV, Latyntseva LD. Evaluation of plasma creatine phosphokinase as a diagnostic dilemma. Russian Medical Journal. 2021;29(2):18-25.
- Fournier S, Iten L, Marques-Vidal P, Boulat O, Bardy D, Beggah A, Calderara R, et al. Circadian rhythm of blood cardiac troponin T concentration. Clin Res Cardiol. 2017;106(12):1026-1032.
doi pubmed - Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97(12):970-976.
doi pubmed - Chaulin AM, Duplyakov DV. MicroRNAs in atrial fibrillation: pathophysiological aspects and potential biomarkers. Int J Biomed. 2020;10:198-205.
doi - Chaulin AM, Duplyakov DV. On the potential effect of circadian rhythms of cardiac troponins on the diagnosis of acute myocardial infarction. Signa Vitae. 2021;17:79-84.
doi - Kapiotis S, Jilma B, Quehenberger P, Ruzicka K, Handler S, Speiser W. Morning hypercoagulability and hypofibrinolysis. Diurnal variations in circulating activated factor VII, prothrombin fragment F1+2, and plasmin-plasmin inhibitor complex. Circulation. 1997;96(1):19-21.
doi pubmed - Montagnana M, Salvagno GL, Lippi G. Circadian variation within hemostasis: an underrecognized link between biology and disease? Semin Thromb Hemost. 2009;35(1):23-33.
doi pubmed - Chaulin AM, Duplyakov DV. Cardiac troponins: Current data on the diagnostic value and analytical characteristics of new determination methods. Cor Vasa. 2021;63:486-493.
doi - Zenina OY, Makarova II, Ignatova YP, Aksenova AV. Chronophysiology and chronopathology of cardiovascular system (literature review). Human Ecol. 2016;1:25-33.
doi - Tsareva YO, Mayskova EA, Fedotov EA, Shvarts YG. [Circadian rhythms of thyroid hormones in patients with ischemic heart disease, arterial hypertension, and atrial fibrillation]. Kardiologiia. 2019;59(3S):23-29.
doi pubmed - Chaulin AM, Grigorieva JV, Suvorova GN, Duplyakov DV. Experimental modeling of hypothyroidism: principles, methods, several advanced research directions in cardiology. Russ Open Med J. 2021;10:e0311.
doi - Michailovich Chaulin A. Current understanding of cardiac troponins metabolism: a narrative review. Curr Med Chem. 2022.
doi pubmed - Hessel MH, Atsma DE, van der Valk EJ, Bax WH, Schalij MJ, van der Laarse A. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Arch. 2008;455(6):979-986.
doi pubmed - Chaulin AM. Phosphorylation and fragmentation of the cardiac troponin T: mechanisms, role in pathophysiology and laboratory diagnosis. Int J Biomed. 2021;11:250-259.
doi - Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WK. Posttranslational modifications of cardiac troponin T: an overview. J Mol Cell Cardiol. 2013;63:47-56.
doi pubmed - Ali SA, Kazmi S, Jalal-Ud-Din M, Qasim MI, Jadoon ZG. Frequency of elevated troponin T in patients of hronic renal failure without clinically suspected acute myocardial infarction. J Ayub Med Coll Abbottabad. 2019;31(3):364-367.
- Long B, Belcher CN, Koyfman A, Bronner JM. Interpreting troponin in renal disease: A narrative review for emergency clinicians. Am J Emerg Med. 2020;38(5):990-997.
doi pubmed - Zou L, Sun W. Human urine proteome: a powerful source for clinical research. Adv Exp Med Biol. 2015;845:31-42.
doi pubmed - Maeda H, Michiue T, Zhu BL, Ishikawa T, Quan L. Analysis of cardiac troponins and creatine kinase MB in cerebrospinal fluid in medicolegal autopsy cases. Leg Med (Tokyo). 2009;11(Suppl 1):S266-268.
doi pubmed - Wang Q, Michiue T, Ishikawa T, Zhu BL, Maeda H. Combined analyses of creatine kinase MB, cardiac troponin I and myoglobin in pericardial and cerebrospinal fluids to investigate myocardial and skeletal muscle injury in medicolegal autopsy cases. Leg Med (Tokyo). 2011;13(5):226-232.
doi pubmed - Chaulin AM. Diagnostic considerations and analytical characteristics of methods for the determination of cardiac troponins: traditional review. Turkiye Klinikleri J Cardiovasc Sci. 2021;33(3):149-160.
doi - Katrukha IA, Kogan AE, Vylegzhanina AV, Serebryakova MV, Koshkina EV, Bereznikova AV, Katrukha AG. Thrombin-mediated degradation of human cardiac troponin T. Clin Chem. 2017;63(6):1094-1100.
doi pubmed - Derhaschnig U, Testori C, Riedmueller E, Aschauer S, Wolzt M, Jilma B. Hypertensive emergencies are associated with elevated markers of inflammation, coagulation, platelet activation and fibrinolysis. J Hum Hypertens. 2013;27(6):368-373.
doi pubmed - Bunin VA, Kozlov KL, Linkova NS, Paltseva EM. An increase in troponin-I concentration in the saliva of patients with coronary heart disease correlates with the stage of disease development. Kompleksnye Problemy Serdecno-Sosudistyh Zabolevanij. 2017;6(S 4):13-14. (in Russian).
- Duque-Ossa LC, Garcia-Ferrera B, Reyes-Retana JA. Troponin I as a biomarker for early detection of acute myocardial infarction. Curr Probl Cardiol. 2021:101067.
doi pubmed - Arshad MK, Bin Mohamad Fathil MF, Gopinath SC, Ruslinda AR, Md Nor MN, Lam HY, Hashim U. Cardiac biomarkers: invasive to non-invasive assessments. Curr Med Chem. 2016;23(37):4270-4284.
doi pubmed - Joharimoghadam A, Tajdini M, Bozorgi A. Salivary B-type natriuretic peptide: a new method for heart failure diagnosis and follow-up. Kardiol Pol. 2017;75(1):71-77.
doi pubmed - Chaulin AM, Duplyakov DV. Increased natriuretic peptides not associated with heart failure. Russian Journal of Cardiology. 2020;25(4S):4140.
doi - Acosta G, Amro A, Aguilar R, Abusnina W, Bhardwaj N, Koromia GA, Studeny M, et al. Clinical determinants of myocardial injury, detectable and serial troponin levels among patients with hypertensive crisis. Cureus. 2020;12(1):e6787.
doi - Pattanshetty DJ, Bhat PK, Aneja A, Pillai DP. Elevated troponin predicts long-term adverse cardiovascular outcomes in hypertensive crisis: a retrospective study. J Hypertens. 2012;30(12):2410-2415.
doi pubmed - Talha Ayub M, Torres C, Del Cid J, Khan MS, Rasool W, Talha Aijaz, et al. The prognostic significance of highly sensitive cardiac troponin i elevation in patients presenting with hypertensive crisis. Circulation. 2019;140:A16333.
- Ravichandran J, Woon SY, Quek YS, Lim YC, Noor EM, Suresh K, Vigneswaran R, et al. High-sensitivity cardiac troponin I levels in normal and hypertensive pregnancy. Am J Med. 2019;132(3):362-366.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


