| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 14, Number 3, March 2022, pages 136-141
Acute Promyelocytic Leukemia in a Patient With Chronic Continuous Type of Crohn’s Disease
Shiori Kinoshitaa, Satoshi Tanidab, d, Akimi Kawaic, Kazuhide Shiragaa, Tomoyuki Nakamurac, Kana Oiwac, Tomotaka Suzukic, Asahi Itoc, Masaki Ric, Shigeru Kusumotoc, Hirokazu Komatsuc, Shinsuke Iidac
aDepartment of Hematology, Toyokawa City Hospital, Toyokawa City, Aichi Prefecture, Japan
bDepartment of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, Nagoya City, Aichi Prefecture, Japan
cDepartment of Hematology and Oncology, Nagoya City University Graduate School of Medical Sciences, Nagoya City, Aichi Prefecture, Japan
dCorresponding Author: Satoshi Tanida, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, Nagoya City, Aichi Prefecture, Japan
Manuscript submitted February 2, 2022, accepted February 26, 2022, published online March 25, 2022
Short title: APL in a Patient With Crohn’s Disease
doi: https://doi.org/10.14740/jocmr4675
| Abstract | ▴Top |
Chronic inflammation can induce leukemogenic mutations in hematopoietic stem cells (HSCs). We report a case of acute promyelocytic leukemia (APL) in a patient with chronic continuous type of Crohn’s disease. The patient had been diagnosed with Crohn’s disease at the age of 28 years and had received conventional treatments with biologics, but not azathioprine. At the age of 51, he was diagnosed with APL with ider(17). Long-term exposure to chronic continuous inflammation from Crohn’s disease might be a factor inducing genomic instability in HSCs, which lead to the subsequent development of APL. APL is a rare hematological manifestation that required attention in Crohn’s disease patients.
Keywords: Acute promyelocytic leukemia; Crohn’s disease; Inflammatory bowel disease; Chronic inflammation; Hematopoietic stem cell; Myeloid neoplasm
| Introduction | ▴Top |
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) that is characterized by the specific chromosomal rearrangement, t(15;17)(q22;q21) [1]. In the past decade, owing to the introduction of all-trans retinoic acid (ATRA) therapy in combination with arsenic trioxide and other chemotherapies, APL has become one of the most curable leukemias [2]. Chromosomal rearrangements in addition to t(15;17) have been reported in 25-40% of APL patients, with a preponderance of trisomy 8 [3]. Other abnormalities are far less frequent, particularly ider(17) [4, 5].
Since the first suggestion of the association between chronic inflammation and tumorigenesis, accumulating evidences have shown that recurrent and persistent chronic inflammation might be a factor in oncogenesis [6, 7]. Chronic inflammation is known to drive the development of cancer [6]. Cytokines, chemokines, growth factors and other immune regulating proteins produced during chronic inflammation can serve as stimuli for the production of pro-survival molecules, leading to DNA damage and instability [8]. In addition, chronic inflammation can affect hematopoietic stem cells (HSCs) and promote genomic instability, potentially leading to the acquisition of leukemogenic mutations [9].
Inflammatory bowel diseases (IBDs), such as ulcerative colitis (UC) and Crohn’s disease, often affect the entire digestive system and organs, and are associated with an increased risk of cancers, including hematological malignancies. In particular, an almost six times higher association between IBD and hematological malignancies has been reported in patients with Crohn’s disease as compared to the general population [10]. There are a few reports of secondary APL in patients with Crohn’s disease [11-15].
The therapeutic treatments for moderate-to-severe Crohn’s disease include 5-aminosalicylic acid (5-ASA), corticosteroids, thiopurines, and biologics including tumor necrosis factor (TNF)-α inhibitors and anti-interleukin (IL)-12/23 antibodies. Thiopurines are known to be associated with an increased risk of lymphomas and myeloid neoplasms, but 5-ASA, corticosteroids and biologics have no association with hematological malignancies [16].
Here, we report a rare case of APL in a patient with a chronic continuous state of Crohn’s disease, who had received Crohn’s disease treatment with 5-ASA, budesonide and various biologics, but not thiopurines.
| Case Report | ▴Top |
Investigations
The patient was a male who had been diagnosed with ileocolitis type of Crohn’s disease at the age of 28 years and was started on 5-ASA and an elementary diet (Elental®) including amino acids, very little fat, vitamins, trace elements, and a major energy source, dextrin which results in reductions in immune stimulation in the gut [17]. However, due to refractoriness to conventional treatment, infliximab (IFX) therapy was added at the age of 40 years. He underwent Hartman’s operation and sigmoid colostomy at the age of 41 years because of sigmoid colon stenosis and formation of a fistula between the bladder and sigmoid colon. Subsequently, high levels of C-reactive protein (CRP) were continuously observed due to persistent active colitis. Repeated follow-up colonoscopy showed active ulcers at the site of anastomosis and in the colon. At the age of 50 years, IFX was switched to ustekinumab, and budesonide was added due to refractory Crohn’s disease with chronic continuous disease activity, for which IFX was not effective. One year later, the patient underwent partial ileectomy because of acute hemorrhage from an ileal Crohn’s disease ulcer.
Soon after the operation, neutropenia and thrombocytopenia were observed. Initially, suspecting 5-ASA-induced bone marrow suppression, the drug was immediately discontinued. With this, although thrombocytopenia improved, neutropenia persisted. Bone marrow aspiration was subsequently performed to diagnose hematological disorders. The first bone marrow evaluation showed hypocellular marrow without an increase in blasts or dysplasia. G-banding analysis of bone marrow showed a normal karyotype. Based on the above, the cause of leukocytopenia was deemed equivocal, and the patient was monitored without hematological treatment.
Diagnosis
After 4 months, bone marrow aspiration was performed again due to gradual worsening of neutropenia. Laboratory investigations at the time of the second bone marrow aspiration showed: white blood cell count, 0.7 × 109/L; blasts, 1.0%; neutrophils, 63.0%; lymphocytes, 36.0%; red blood cell count, 3.15 × 1012/L; hemoglobin, 10.9 g/dL; platelet count, 154 × 109/L; Fe, 70 µg/dL; unsaturated iron biding capacity (UIBC), 469 µg/dL; ferritin, 10 ng/mL; CRP, 0.27 mg/dL; D-dimer, 14.5 µg/dL and fibrin degradation products; 37.7 mg/dL (Table 1). Iron deficiency anemia was diagnosed which was presumed to be due to decreased levels of iron absorption secondary to active Crohn’s disease. The second bone marrow aspiration displayed hypocellular marrow with significant increases in blasts and promyelocytes, up to 26.8% and 12%, respectively. Some blasts consisted of basophilic cells with cytoplasmic projections. There were many promyelocytes with numerous azurophil granules positive for myeloperoxidase stain (Fig. 1). Flow cytometry analysis showed that the blasts were positive for CD13 and CD33; negative for CD34 and human leukocyte antigen (HLA)-DR. Cytogenetic studies showed a karyotype of 46, XY, der(15)t(15;17)(q24.1:q21.2), ider(17)(q10)t (15;17) (Fig. 2). Using fluorescence in situ hybridization, the promyelocytic leukemia/retinoic acid receptor α (PML/RARA) fused gene signal was positively detected in 76% of the cells. In addition, quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis for the PML-RARA rearrangement showed 35,000 copies/µg RNA. Based on the above findings, the patient was diagnosed with APL.
 Click to view | Table 1. Laboratory Data at the Time of the Second Bone Marrow Aspiration |
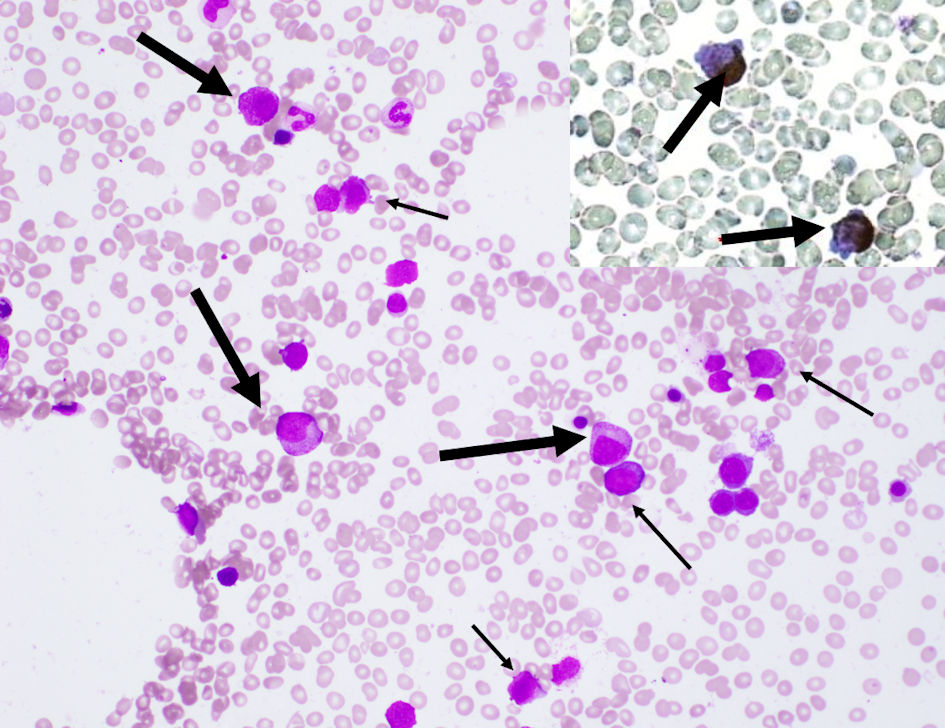 Click for large image | Figure 1. Bone marrow aspiration showed a hypocellular marrow with increased blasts (narrow arrows), and promyelocytes (wide arrows) with azurophil granules that were positive on peroxidase staining (upper right box) (magnification, × 400). |
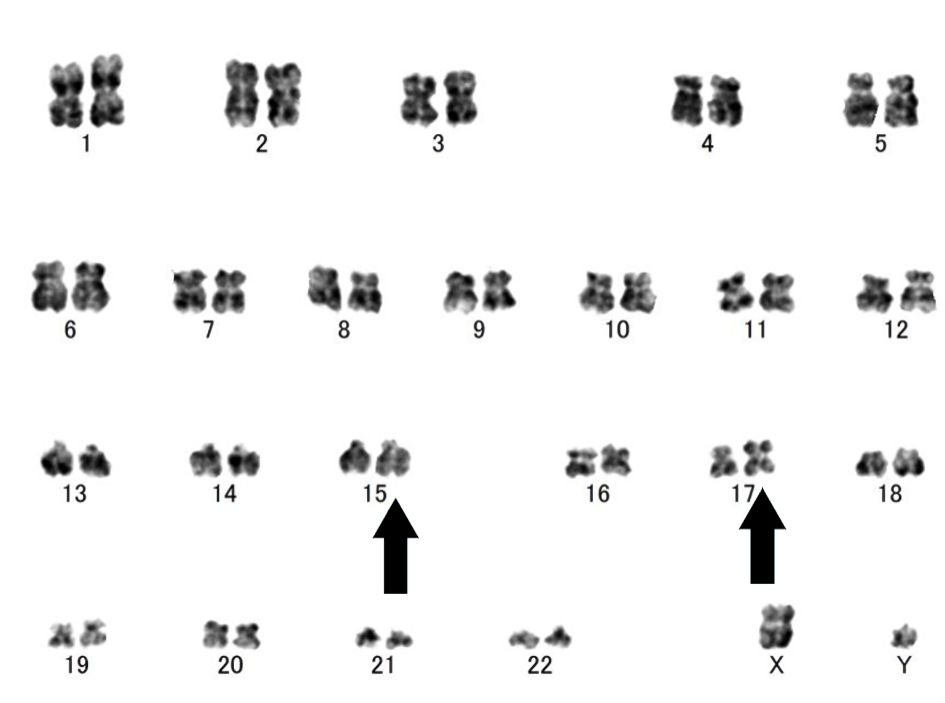 Click for large image | Figure 2. G-banding at the time of diagnosis of acute promyelocytic leukemia (APL) showed a karyotype of 46, XY, der(15)t(15;17)(q24.1:q21.2), ider(17)(q10)t(15;17) (arrows). |
Treatment
According to the Japan adult leukemia study group-204 protocol, induction chemotherapy with ATRA at a dose of 45 mg/m2 was started. With this, complete hematological remission was achieved after 31 days, although qPCR of PMR/RARA was still positive, at 28,000 copies/µg. Consolidation therapy with 7 mg/m2/day of mitoxantrone for 3 days and 200 mg/m2/day of cytarabine for 5 days was subsequently administered. The qPCR value of PML-RARA decreased to undetectable levels after one cycle of consolidation therapy and complete molecular remission was achieved. Bone marrow aspiration findings after the consolidation therapy showed hypocellular marrow with no apparent blast cell abnormalities or dysplasia. The second cycle of consolidation consisted of 50 mg/m2/day of daunorubicin for 3 days and cytarabine 200 mg/m2/day for 5 days. Although the complete molecular remission was sustained, it took 70 days until bone marrow recovery. Bone marrow aspiration after the second cycle of consolidation therapy showed hypocellular marrow and binuclear erythroblasts. G-banding analysis of bone marrow showed a normal karyotype. A consolidation regimen with 0.15 mg/kg of arsenic trioxide for 25 days was administered because of the concerns about a high risk of infection due to severe and persistent myelosuppression.
Follow-up and outcomes
The patient is currently still in remission, although complete recovery of bone marrow was not achieved for 3 months after the last consolidation.
| Discussion | ▴Top |
Here, we report a case of APL in a patient who had been in a state of active Crohn’s disease for 23 years and had received conventional treatments, including 5-ASA, corticosteroids and various biologics, but excluding azathioprine.
A large population-based study on therapeutic treatments for immune-mediated diseases strongly implicated azathioprine as a causative agent of myeloid neoplasms [16]. The leukemogenic mechanism of azathioprine is considered to involve prevention of repair of drug-induced DNA double-strand breaks [18], and induction of mismatch repair (MMR) deficiency, as reflected by microsatellite instability [19, 20]. On the other hand, conventional treatments and biologics, including anti-TNF-α antagonists and ustekinumab, reportedly only have a slight association with myeloid neoplasms in patients with IBD and other immune-mediated diseases [16, 21-26]. However, our patient developed APL even though he had not received thiopurines. Four patients of six previous APL cases associated with Crohn’s disease received conventional treatment without thiopurines and another patient received multiple small bowel resections and colostomy (Table 2) [11-15]. Only one patient was treated with thiopurines. This suggests that other factors which are little associated with Crohn’s disease treatment probably induce APL development. Our patient underwent intestinal partial resections twice because persistent active Crohn’s disease colitis, ileac ulcers and high levels of CRP value were continuously observed. Chronic active Crohn’s disease inflammation causing repeated intestinal resections might have induced APL development.
 Click to view | Table 2. Acute Promyelocytic Leukemia (APL) in Crohn’s Disease Patients |
An increased incidence of AML has been reported in patients with several immune-mediated diseases and IBD [27-29]. A nationwide historical cohort study also demonstrated that Crohn’s disease was closely associated with hematological malignancies [30]. These observations suggest that chronic inflammation underlying immune-mediated diseases might be a causative factor in the development of myeloid neoplasms.
Continuous production of proinflammatory cytokines mediating chronic inflammation, such as IL-1β, TNF-α and interferons, have been reported to lead to significant alterations in HSC function and output [31]. Furthermore, chronic inflammation might augment exposure of HSCs to oxidative stress conditions from reactive oxygen species in the bone marrow niche, which maintain and regulate HSCs and play an important role in the pathogenesis of hematological malignancies [32]. These stresses often promote genomic instability and, potentially, the acquisition and increasing accumulation of leukemogenic mutations in HSCs [9, 33]. Therefore, chronic inflammation might function as an initiator and driver of myeloid neoplasms [34]. In addition, a recent study suggested that a long disease duration of IBD might induce MMR deficiency [35]. Thus, MMR deficiency during chronic inflammation in IBD might be another factor that promotes genomic instability in HSCs and causes myeloid neoplasms.
In conclusion, our patient developed APL although he had never received thiopurines which are known to be associated with an increased risk of development of myeloid neoplasms. To the best of our knowledge, there are a few case reports of APL in patients with Crohn’s disease [11-15]. Although further investigations are needed to certify the association between Crohn’s disease and APL occurrence, careful attention should be paid to the development of myeloid neoplasms in Crohn’s disease patients with long-term active inflammation.
Acknowledgments
None to declare.
Financial Disclosure
There has been no significant financial support for this work.
Conflict of Interest
Shinsuke Iida has received honoraria from Janssen Pharmaceutical K.K. and research funding from Janssen Pharmaceutical K.K. and Pfizer Inc. Shigeru Kusumoto has received honoraria from Chugai Pharmaceutical Co., Ltd. and research funding from Daiichi Sankyo Co., Ltd. Other authors have no conflict of interest to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
SK prepared and wrote this manuscript. SK, ST and AK contributed to clinical management of this patient, collection and review of patient’s chart. ST and HK contributed to reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this report are available within the article.
Abbreviations
HSCs: hematopoietic stem cells; APL: acute promyelocytic leukemia; AML: acute myeloid leukemia; ATRA: all-trans retinoic acid; IBDs: inflammatory bowel diseases; UC: ulcerative colitis; 5-ASA: 5-aminosalicylic acid; TNF: tumor necrosis factor; IFX: infliximab; CRP: C-reactive protein; UIBC: unsaturated iron biding capacity; HLA: human leukocyte antigen; PML/RARA: promyelocytic leukemia/retinoic acid receptor α; qRT-PCR: quantitative reverse transcriptase-polymerase chain reaction; MMR: mismatch repair
| References | ▴Top |
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Swerdlow SH. International Agency for Research on C. WHO classification of tumours of haematopoietic and lymphoid tissues. rev. 4th ed: International Agency for Research on Cancer. 2017. p. 585.
- Cervera J, Montesinos P, Hernandez-Rivas JM, Calasanz MJ, Aventin A, Ferro MT, Luno E, et al. Additional chromosome abnormalities in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Haematologica. 2010;95(3):424-431.
doi pubmed - He Y, Wang P, Liang K, Chen X, Du W, Li J, Hu Y, et al. A pediatric acute promyelocytic leukemia with a rare karyotype of ider(17)(q10)t(15;17) and favorable outcome: a case report. Medicine (Baltimore). 2015;94(41):e1778.
doi pubmed - Liu Y, Xu J, Chu L, Yu L, Zhang Y, Ma L, Wang W, et al. A rare case of acute promyelocytic leukemia with ider(17)(q10)t(15;17)(q22;q21) and favorable outcome. Mol Cytogenet. 2020;13:13.
doi pubmed - Moss SF, Blaser MJ. Mechanisms of disease: Inflammation and the origins of cancer. Nat Clin Pract Oncol. 2005;2(2):90-97.
doi pubmed - Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539-545.
doi - Pua KH, Chew CL, Lane DP, Tergaonkar V. Inflammation-associated genomic instability in cancer. Genome Instability & Disease. 2020;1(1):1-9.
doi - Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, Hoogenboezem RM, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. 2016;19(5):613-627.
doi pubmed - So J, Tang W, Leung WK, Li M, Lo FH, Wong MTL, Sze ASF, et al. Cancer risk in 2621 Chinese patients with inflammatory bowel disease: a population-based cohort study. Inflamm Bowel Dis. 2017;23(11):2061-2068.
doi pubmed - Mohammad F, Vivekanandarajah A, Haddad H, Shutty CM, Hurford MT, Dai Q. Acute promyelocytic leukaemia (APL) in a patient with Crohn's disease and exposure to infliximab: a rare clinical presentation and review of the literature. BMJ Case Rep. 2014;2014:bcr2013203318.
doi pubmed - Takitani K, Inoue A, Kawakami C, Miyazaki H, Aomatsu T, Yoden A, Suzuki K, et al. Reduced plasma all-trans retinoic acid level in a patient with Crohn's disease with acute promyelocytic leukemia. Leuk Lymphoma. 2009;50(2):300-302.
doi pubmed - Crispino P, Pica R, Angelucci E, Consolazio A, Rivera M, Cassieri C, Paoluzi P. Hematological malignancies in chronic inflammatory bowel diseases: report of five cases and review of the literature. Int J Colorectal Dis. 2007;22(5):553-558.
doi pubmed - Harewood G, Markovic S. Treatment of acute myeloid leukemia M3 in a patient with Crohn's disease. Cancer Invest. 2000;18(1):98.
doi pubmed - Orii S, Sugai T, Nakano O, Yoshinari H, Sato S. Acute promyelocytic leukemia in Crohn's disease. Case report and review of the literature. J Clin Gastroenterol. 1991;13(3):325-327.
doi pubmed - Ertz-Archambault N, Kosiorek H, Taylor GE, Kelemen K, Dueck A, Castro J, Marino R, et al. Association of therapy for autoimmune disease with myelodysplastic syndromes and acute myeloid leukemia. JAMA Oncol. 2017;3(7):936-943.
doi pubmed - O'Morain C, Segal AW, Levi AJ. Elemental diet as primary treatment of acute Crohn's disease: a controlled trial. Br Med J (Clin Res Ed). 1984;288(6434):1859-1862.
doi pubmed - Kwong YL, Au WY, Liang RH. Acute myeloid leukemia after azathioprine treatment for autoimmune diseases: association with -7/7q. Cancer Genet Cytogenet. 1998;104(2):94-97.
doi - Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, Burke MM, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104(3):822-828.
doi pubmed - Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92(10):1389-1398.
doi pubmed - Charakopoulos E, Spyrou I, Viniou NA, Giannakopoulou N, Hatzidavid S, Diamantopoulos PT. A case report of Hodgkin lymphoma in a patient treated with ustekinumab for psoriasis. Medicine (Baltimore). 2020;99(21):e20048.
doi pubmed - Gonzalez-Ramos J, Alonso-Pacheco ML, Mayor-Ibarguren A, Herranz-Pinto P. Gastric mucosa-associated lymphoid tissue lymphoma in a patient with severe psoriasis receiving ustekinumab. Actas Dermosifiliogr. 2015;106(4):326-327.
doi pubmed - Humme D, Beyer M, Rowert-Huber HJ, Sterry W, Philipp S. [CD30-positive anaplastic large cell T-cell lymphoma developing during immunosuppressive therapy of pityriasis rubra pilaris with ustekinumab]. Hautarzt. 2013;64(3):190-194.
doi pubmed - Young L, Czarnecki D. The rapid onset of multiple squamous cell carcinomas in two patients commenced on ustekinumab as treatment of psoriasis. Australas J Dermatol. 2012;53(1):57-60.
doi pubmed - Ehmann LM, Tillack-Schreiber C, Brand S, Wollenberg A. Malignant melanoma during ustekinumab therapy of Crohn's disease. Inflamm Bowel Dis. 2012;18(1):E199-200.
doi pubmed - Rao RR, Majlessipour F, Ziring DA, Baca NM. Ewing's sarcoma in a patient with Crohn's disease treated with Ustekinumab: a case report. J Adolesc Young Adult Oncol. 2021;10(5):614-617.
doi pubmed - Ramadan SM, Fouad TM, Summa V, Hasan S, Lo-Coco F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012;97(6):805-817.
doi pubmed - Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822-828.
doi pubmed - Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897-2903.
doi pubmed - Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sorensen HT, Baron JA. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12(2):265-273.e261.
doi pubmed - King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685-692.
doi pubmed - Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1-2):7-25.
- Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T, Wagers AJ, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285-299.
doi pubmed - Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130(15):1693-1698.
doi pubmed - Kuehn F, Klar E, Bliemeister A, Linnebacher M. Reactivity against microsatellite instability-induced frameshift mutations in patients with inflammatory bowel disease. World J Gastroenterol. 2015;21(1):221-228.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


