| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Short Communication
Volume 13, Number 12, December 2021, pages 556-562
Intravascular Ultrasound Can Be Used to Locate Nerves, but not Confirm Ablation, During Renal Sympathetic Denervation
Keisuke Okamuraa, c, Shunsuke Satoua, Yusuke Katob, Yusuke Kogatab, Masatoshi Matsushimaa, Kazuyuki Shiraia, Hidenori Urataa
aDepartment of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, Chikushino, Japan
bOtsuka Medical Devices Co., Ltd, Tokyo, Japan
cCorresponding Author: Keisuke Okamura, Department of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, 1-1-1, Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
Manuscript submitted September 10, 2021, accepted December 20, 2021, published online December 28, 2021
Short title: Intravascular Ultrasound to Locate Nerves
doi: https://doi.org/10.14740/jocmr4597
| Abstract | ▴Top |
Background: No methods exist for confirming nerve ablation in catheter-based renal sympathetic denervation (RDN).
Methods: We investigated the feasibility of using intravascular ultrasound (IVUS) to locate nerves and observe nerve integrity changes during RDN in a pig. To confirm our observations, we used post-RDN histological sections matched anatomically to the IVUS images.
Results: IVUS revealed multiple hypoechoic structures along the renal artery, whose locations matched those of nerves in the histological sections. Nerves clustered near the junction between the renal artery and vein. Histology confirmed necrosis of nerve bundles at RDN ablation sites, but no changes in echogenicity were observed using IVUS.
Conclusions: Although IVUS cannot currently be used to confirm ablation during RDN, it clearly reveals some clusters of renal sympathetic nerves. It remains to be demonstrated how IVUS can guide RDN devices and potentially improve ablation success.
Keywords: Catheter-based renal sympathetic denervation; Renal sympathetic nerves; Intravascular ultrasound
| Introduction | ▴Top |
Renal sympathetic denervation (RDN) is a catheter-based procedure used to ablate sympathetic nerves that run along the renal arteries. The procedure lowers blood pressure, as reported in recent clinical trials such as SPYRAL HTN-ON MED [1], SPYRAL HTN-OFF MED [2], and RADIANCE-HTN SOLO [3]. However, it is not always successful, with approximately one in three patients undergoing RDN showing a lesser blood pressure reduction. One reason for this could be incomplete nerve ablation, and there are no established methods for assessing whether nerve ablation has been achieved. Intraoperatively demonstrating nerve ablation around the renal arteries would confirm procedural success and may reduce the number of non-responders.
We previously reported that renal sympathetic nerves can be imaged using intravascular ultrasound (IVUS) [4]. In the present study, we examined whether renal sympathetic nerves can be visualized with IVUS in a normotensive porcine model, and whether their anatomical positions correspond to those of post-RDN histological sections. We also investigated whether we could observe any changes in the nerves in IVUS images and histological sections before and after RDN (validation 2).
| Materials and Methods | ▴Top |
Validation 1: IVUS visualization of renal sympathetic nerves in a porcine model
Blood vessels in pigs are similar in diameter and anatomical structure to those in humans. Porcine models have been used to assess the safety and efficacy of RDN, including confirming ablation and assessing the impact of the procedure on peripheral tissues [5]. In addition, we used a normotensive porcine model instead of hypertensive porcine model. Because the purpose of study was an anatomical evaluation rather than an evaluation of the antihypertensive effect.
First, we placed a specific pathogen-free, triple-crossbred (LWD) conventional sow (n = 1) under general anesthesia and performed renal arteriography with a guiding catheter (8 Fr, RDC) inserted via the femoral artery (Fig. 1A).
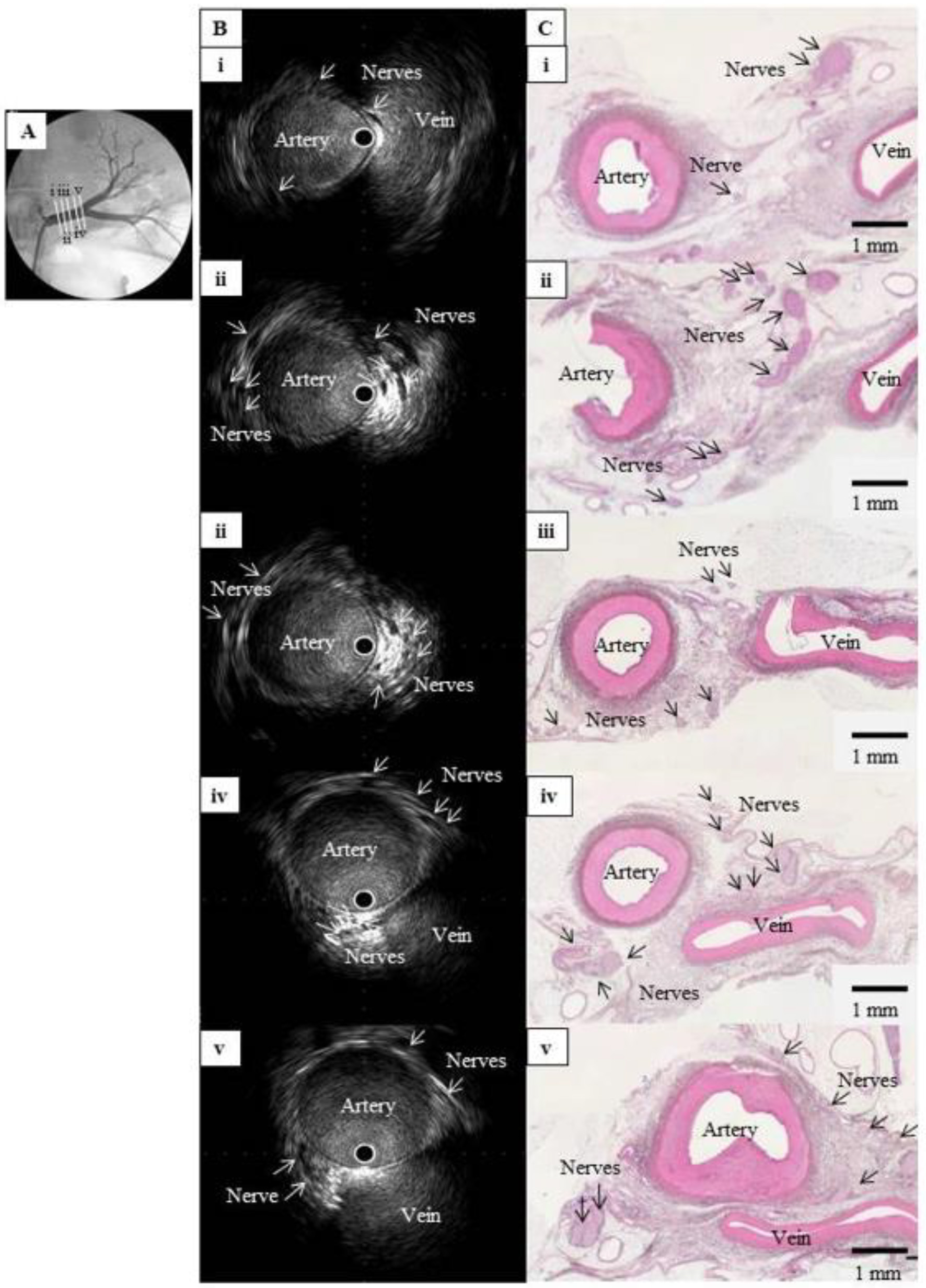 Click for large image | Figure 1. Comparison of IVUS images and histological sections from a normal renal artery in a pig. (A) Left renal angiography with cross-sections of the main renal artery, labeled i (nearest the abdominal aorta) to v (nearest the kidney) and viewed in (B) and (C). (B) Intravascular ultrasound images of the cross-sections in (A), showing multiple hypoechoic regions without luminal structures along the outside of the renal artery. These structures clustered near the junction between the artery and vein. (C) Hematoxylin-eosin-stained histological images of the cross-sections in (A), showing multiple nerve bundles in the connective tissue around the renal artery. The vein appeared considerably atrophied with no blood flow, but the images confirmed that the clustered hypoechoic structures in (B) were nerves. IVUS: intravascular ultrasound; |
We then used IVUS to observe the inside and outside of the blood vessels. The nerves lie closer to the arterial lumen distally, so they must be observed as far as the distal portion of the main renal artery, but advancing the IVUS system too far into the renal vasculature may result in the guidewire or probe damaging the renal parenchyma. To avoid this, we used the NaviFocus® equipped with an IVUS probe, which features a short distance from the tip to the ultrasound element.
For IVUS scanning, the wire was inserted then pulled back to eliminate wire artifact, enabling the outside of the blood vessel to be clearly seen (Fig. 1B). Normally, in ultrasound imaging, the hyperechoic vascular wall appears bright, whereas the renal sympathetic nerves are observed as hypoechoic structures running along the renal artery.
Validation 2A: assessment of spatial changes during and after RDN
First, we examined whether IVUS images would reveal changes to tissue or nerve echogenicity during and after RDN. The Paradise® Ultrasound Denervation System (ReCor Medical Inc., Palo Alto, CA, USA) used in the RADIANCE-HTN trial delivers ultrasound waves to the tissue surrounding the renal artery, providing full-circumference cauterization of nerves to a depth of 1 - 6 mm from the artery [3]. During RDN, the tissue temperature increases to around 70 °C or more [3], water present in the tissue decreases and protein denaturation occurs. Ablated tissues, including nerves, therefore might appear as bright (hyperechoic) areas in IVUS images. We expected that once the IVUS probe was pulled back from the ablation site after RDN, IVUS would provide confirmation of ablation, so we examined the renal artery before and after RDN using the Paradise® system (Fig. 2B).
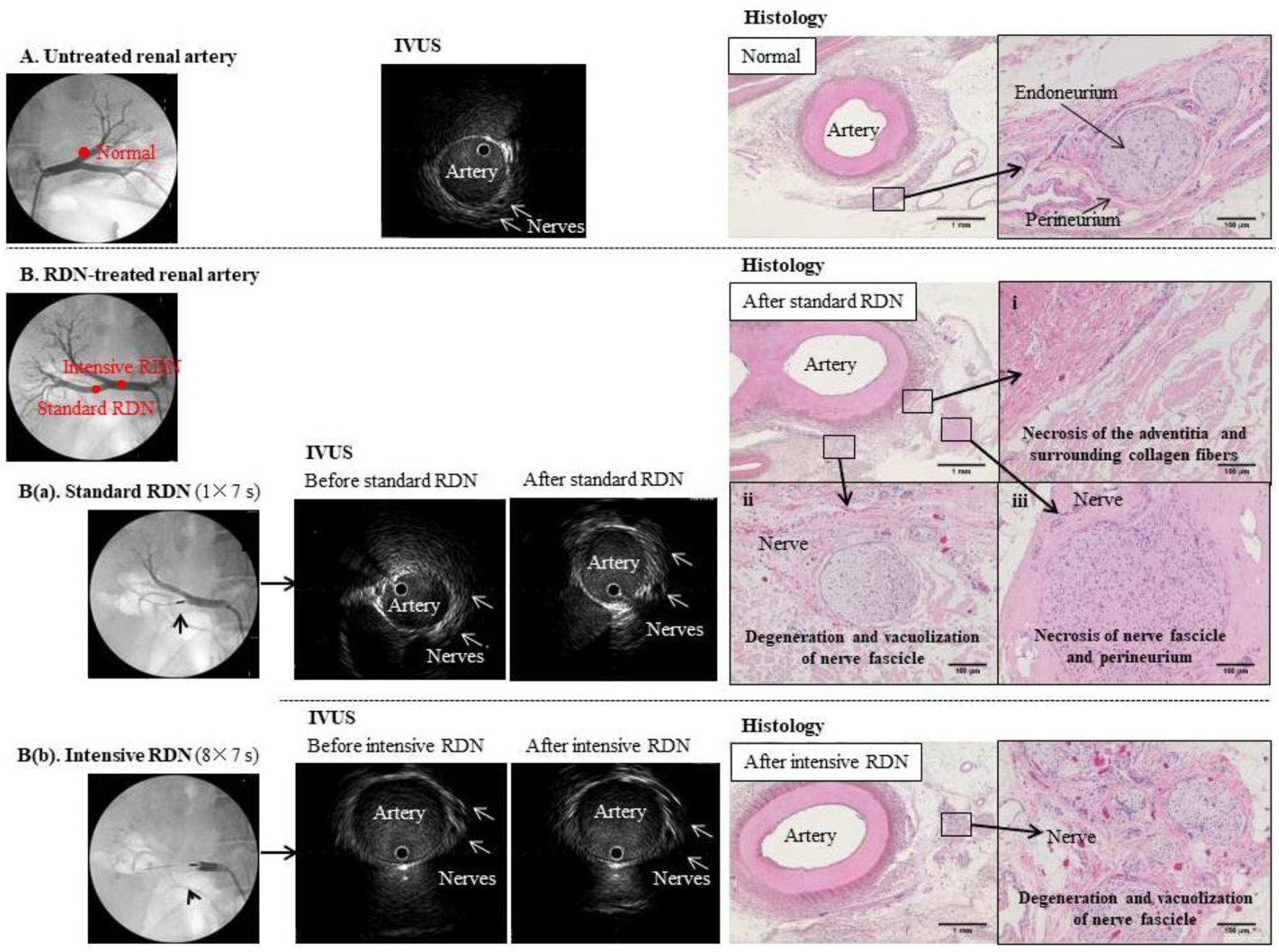 Click for large image | Figure 2. Comparison of IVUS images and tissue specimens before and after RDN. (A) Untreated renal artery. Left panel: Angiogram of normal left renal artery branch. Middle panel: IVUS image of the same artery branch showing hypoechoic structures that appear to be nerves outside the blood vessel. Right panel: hematoxylin-eosin-stained tissue section at the same anatomical level as the IVUS image. Magnified image shows a normal periarterial nerve bundle. Each nerve fiber is covered by the endoneurium (fibrous connective tissue) and gathered into a small bundle, which is surrounded by the perineurium (a sheath of collagenous fibers) and gathered into a fiber bundle. Because the nerve fibers meander inside the perineurium, the cross-sections of individual fibers vary from transverse to almost longitudinal. (B) Renal artery before and after standard (a) and intensive (b) RDN. Left panels: Angiogram showing placement of RDN device in the right renal artery branch. Middle panels: IVUS images showing the artery and surrounding nerves before and after (a) standard RDN (one treatment of 7 s) and (b) intensive RDN (eight treatments of 7 s); multiple hypoechoic structures that appear to be nerves can be seen outside the blood vessels, but no clear changes to these structures were observed even after excessive ablation. Right panels: hematoxylin-eosin-stained sections confirmed ablation after RDN. |
Validation 2B: real-time monitoring of changes during RDN
Next, we sought to confirm whether temporal changes in morphology due to RDN could be observed using IVUS images. We inserted 8-Fr sheaths bilaterally via the inguinal arteries, and two guiding catheters into the renal arteries. The Paradise® system was placed adjacent to the IVUS probe, at the same position in the renal artery, and ablation was performed under IVUS visualization (Fig. 3A, B). Using this method, since the observation and ablation positions are the same, we expected to be able to observe the effects of ablation in real time.
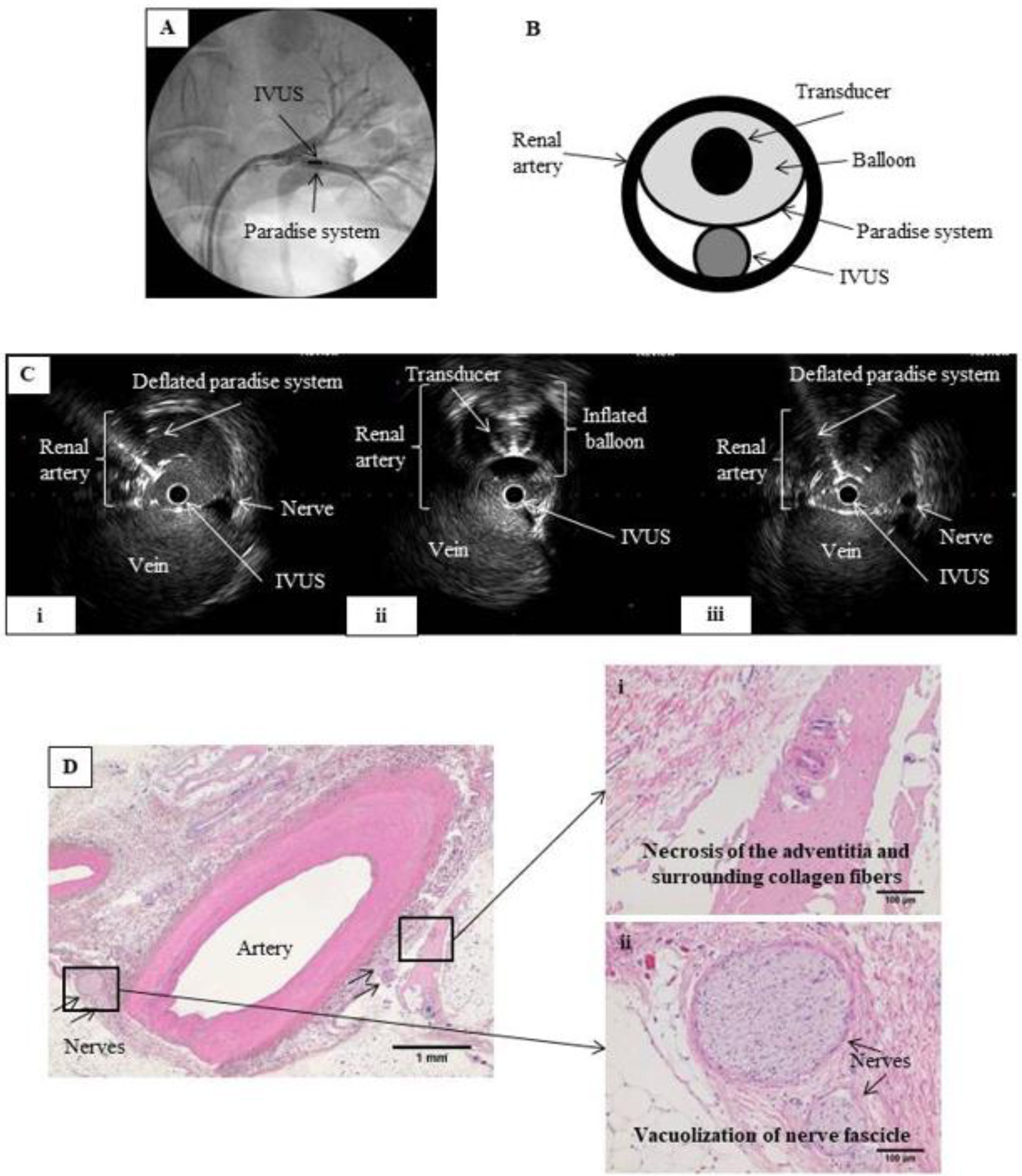 Click for large image | Figure 3. RDN in the renal artery under IVUS monitoring. (A) Renal angiography. An 8 Fr guiding catheter was inserted on each side via the inguinal artery into the left renal artery. The IVUS probe and the Paradise® system transducer were placed parallel to each other at the same point within the renal artery. (B) Schematic of renal artery cross-section showing parallel placement of the IVUS probe and Paradise® system transducer in the artery. Ablation was performed while monitoring with IVUS. (C) IVUS images before, during, and after IVUS-guided RDN. RDN-induced changes in the artery wall can be viewed in real time during the procedure. (i) Before RDN, showing the IVUS probe and the deflated balloon of the Paradise® system inside the renal artery. (ii) During RDN, showing the inflated balloon of the Paradise® system, which appears as a perfect circle. (iii) After RDN, showing the IVUS probe and deflated balloon in the renal artery. We expected that IVUS would allow us to observe the ablation in real time, but no clear differences in nerves were observed before and after the procedure. (D) Hematoxylin-eosin-stained tissue section after IVUS-guided RDN. Magnified images show (i) necrosis of the adventitia and surrounding collagen fibers and (ii) vacuolization of the nerve fascicle, confirming successful RDN. |
On IVUS imaging, the Paradise® system transducer caused a shadow, so visibility was limited in areas behind the Paradise catheter. Likewise, the IVUS catheter shielded tissue behind it from the ablative energy from the Paradise catheter. Therefore, we excluded those areas when evaluating the ablation site before and after RDN.
Validation: histology
The pig was sacrificed, and both renal arteries were excised with the surrounding tissue and fixed in formalin. Paraffin sections (3 µm thick) were prepared and stained with hematoxylin-eosin. We examined the sections histopathologically to determine the distribution of renal arterial sympathetic nerves and the extent of damage after RDN.
The study was approved by IVTeC Institutional Review Board (IVT19-48). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
| Results | ▴Top |
Validation 1: IVUS can be used to locate renal nerves
IVUS images revealed multiple hypoechoic structures along the renal artery, in the range of 1 - 4 mm from the outside wall of the vessel (Fig. 1B). These structures had no lumen, no blood flow detected by Doppler echo, and tended to cluster near the bifurcation of the renal artery or the junction between the artery and vein (Fig. 4A). Nerve fibers generally appear hypoechoic in ultrasound images, and these structures were very similar in arrangement and size to the nerves in renal artery sections imaged by Sakakura et al [6]. Furthermore, the hypoechoic structures in the IVUS images matched the positions of the nerves in the post-RDN histological sections (Figs. 1C and 4B). Together, this evidence indicates that these structures are renal nerves.
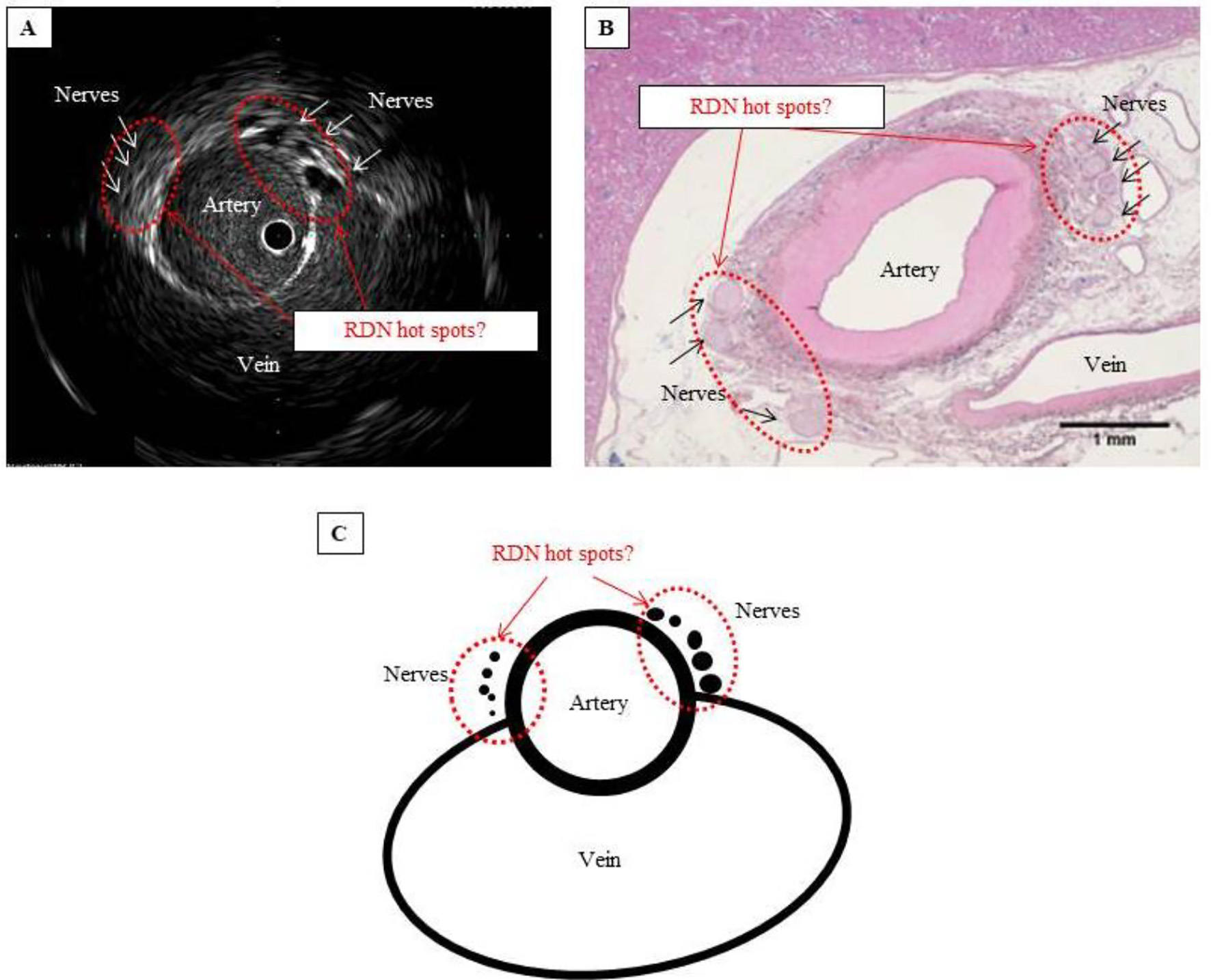 Click for large image | Figure 4. Are these hot spots that should be targeted in RDN? (A) IVUS image showing that the nerves cluster where the artery and vein lie closest to each other. (B) Hematoxylin-eosin-stained tissue section showing considerably atrophied vein, with no blood flow, but confirming the cluster of nerves where the artery and vein lie closest to each other. (C) Schematic of hot spot theory. By selectively performing RDN at a site where the nerves cluster, ablation can be performed efficiently and with pinpoint accuracy. Such a site can be considered a hot spot that potentially improves the blood pressure-lowering effect of RDN. |
The histological sections each contained several nerve bundles. In each section, we measured the distance between the vessel wall and the center of the nerve bundle closest to the blood vessel. The mean distance from the intima of the renal artery to the center of the nerve bundle was 1.6 ± 0.6 mm (12 sites) for the right side and 1.7 ± 0.6 mm (eight sites) for the left side. When these distances were compared with the distance from the entrance of the renal artery to the location of each section, a negative correlation was found for the right (r = -0.688; P = 0.013) and left (r = -0.836; P = 0.010) renal arteries, confirming that the more distal the nerve, the closer it approaches to the renal artery.
Validation 2A: successful ablation was confirmed by histology but not IVUS
For RDN sites where meaningful renal arterial spasm was noted, we performed IVUS observations at 30 min after RDN. When the ablation and non-ablation sites in the artery wall were compared before and after standard RDN (one 7-s ablation), no changes were observed in the IVUS images (Fig. 2B(a), middle panel). To test whether changes to the IVUS image could be observed more distinctly, we carried out an extreme case performing intensive RDN (eight consecutive 7-s ablations; total 56 s) at the same site, but could not observe any changes in the IVUS images (Fig. 2B(b), middle panel).
Conversely, in the pathological tissue specimens containing the sites at which the ablation procedure was performed, degeneration/necrosis due to RDN was observed in the nerve bundles and tissue surrounding the artery at all sites (Fig. 2B(a, b), right panels).
Validation 2B: IVUS did not show real-time changes to nerves during RDN
Although the nerves could be identified, ultrasound interference from the RDN procedure resulted in noisy IVUS images, and no changes to nerves were apparent in these images during and after RDN (Fig. 3C). This is despite degeneration and necrosis of nerve bundles and tissue surrounding the artery being clearly observable at ablation sites in the histological sections.
| Discussion | ▴Top |
The porcine model is similar in size, length, and anatomy of the human renal artery. Since the porcine model has been used in many RDN studies so far, we examined it this examination as well. In addition, the usefulness of the porcine study is that it can be dissected after the procedure and the actual ablation status can be confirmed immediately.
Validation 1: IVUS reveals RDN “hot spots”
Comparing IVUS images with histological images confirmed that the hypoechoic regions observed in the IVUS images were renal nerves. The location of the renal sympathetic plexus is usually reported as 0.5 - 1.0 mm [7] or 2.0 - 8.0 mm [6] from the renal artery lumen. We measured the mean distance from the renal artery lumen as 1.6 - 1.7 mm in our histological sections, but since the sections had no vascular blood and were deformed by formalin fixation, we believe the distance under physiological conditions would be much greater.
Some nerves join the renal artery more distally in the main renal artery and/or travel closer to the distal portion of the main renal artery than the proximal portion. Therefore, for reducing blood pressure, RDN ablation is recommended in the distal portion of the main renal artery [6]. Consistent with this, measurements from our histological sections confirmed that the more distal the nerves, the closer they run to the renal arterial wall.
Furthermore, we were able to confirm both histopathologically and in IVUS images that sympathetic nerves cluster near the junction between the renal artery and vein (Fig. 4A, B). This may serve as a “hot spot” for RDN, where the blood pressure-lowering capability of RDN is enhanced if ablation is selectively performed at this location (Fig. 4C). We posit that ablation at sites other than this hot spot may explain non-responder cases, in which RDN fails to lower blood pressure. One method for determining the target site for RDN has been suggested as electrical autonomic nervous stimulation from the inner part of the renal artery [8], and we suspect this relates to the anatomical site of the hot spot.
Anatomical factors relating to hot spots and ablation sites might contribute to the success or failure of RDN as a blood pressure-lowering procedure. When performing circumferential ultrasound ablation, positioning the device at a hot spot under IVUS guidance may help achieve successful ablation because the entire circumference is treated at once. However, with a standard ultrasound device, ablation may be more difficult, since the nerve hot spot is located close to the vein, and venous blood flow might cool the hot spot, preventing a sufficient temperature increase for ablation. This warrants further investigation.
Validation 2: IVUS cannot be used to confirm ablation
Despite the tissue specimens clearly showing RDN-induced nerve ablation, we could not definitively confirm the extent of ablation from the IVUS images even after intensive RDN. We suspect that this is due to the resolution of IVUS being too low for the small diameter of the nerves. While there will be a degree of inter-individual variation in nerve diameter, our results suggest that IVUS may not be the most appropriate method of confirming ablation in a clinical context.
Conclusion
We observed spatial and temporal changes in renal nerves after RDN in both IVUS images and histological sections, but no clinically useful, real-time changes could be detected when IVUS was used during the RDN procedure. However, hot spots of renal sympathetic nerves were visible with IVUS, suggesting that IVUS could be used to guide RDN devices to these locations and improve the rate of successful ablation in RDN procedures.
Limitations
In this study, only one pig was used. For one location, the RDN catheter was used concurrently with the IVUS catheter in same site of the artery, there were two locations where ablations were attempted a total of eight times (56 s) at same site, and some of the ablations were done distal to the bifurcation of the main renal artery. All of the three experimental usages were different to how the device is used clinically. It may also be possible that ultrasound waves from the Paradise® system damaged the IVUS probe and vice versa, when these were used concurrently.
Acknowledgments
This study was funded by Otsuka Medical Devices. The animal experiment was performed by the IVTeC Corporation Kobe Lab, and the tissue specimen analysis was performed by the New Drug Research Center Inc.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have received honoraria and animal experiment opportunities, as well as grant support for a clinical trial of a treatment for resistant hypertension with an ultrasonic RDN system, from Otsuka Medical Devices Co., Ltd. Yusuke Kato and Yusuke Kogata are employees of Otsuka Medical Devices Co., Ltd.
Informed Consent
Not applicable.
Author Contributions
Keisuke Okamura, Yusuke Kato, and Yusuke Kogata: study design, data collection, data interpretation, and manuscript preparation; Shunsuke Satou: data collection and data interpretation; Masatoshi Matsushima: data interpretation; Kazuyuki Shirai and Hidenori Urata: manuscript preparation.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346-2355.
doi - Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160-2170.
doi - Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335-2345.
doi - Satou S, Okamura K, Konishi R, Shirai K, Urata H. Observation of renal sympathetic nerves by intravascular ultrasound. Hypertens Res. 2019;42(7):1092-1094.
doi pubmed - Sakakura K, Roth A, Ladich E, Shen K, Coleman L, Joner M, Virmani R. Controlled circumferential renal sympathetic denervation with preservation of the renal arterial wall using intraluminal ultrasound: a next-generation approach for treating sympathetic overactivity. EuroIntervention. 2015;10(10):1230-1238.
doi pubmed - Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64(7):635-643.
doi pubmed - Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat. 2012;25(5):628-633.
doi pubmed - Chinushi M, Izumi D, Iijima K, Suzuki K, Furushima H, Saitoh O, Furuta Y, et al. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61(2):450-456.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


