| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 6, June 2022, pages 219-228
Analgesic Efficacy of Multiple Single-Shot Peripheral Nerve Blocks on Postoperative Short-Term Opioid Usage and Clinical Outcomes in a Suburban Hospital Setting
James Solera, Ned Sciortinob, Sara Badaglialacquac, Craig Ryand, Greg Marchandd, e
aUniversity of Arizona College of Nursing, Tucson, AZ, USA
bMidwestern School of Osteopathic Medicine, Glendale, AZ, USA
cMidwestern School of Pharmacy, Glendale, AZ, USA
dMarchand Institute for Minimally Invasive Surgery, Mesa, AZ, USA
eCorresponding Author: Greg J. Marchand, Marchand Institute for Minimally Invasive Surgery, Mesa, AZ 85209, USA
Manuscript submitted April 28, 2022, accepted June 9, 2022, published online June 27, 2022
Short title: Single-Shot Suburban Cohort
doi: https://doi.org/10.14740/jocmr4731
| Abstract | ▴Top |
Background: Preoperative single-shot peripheral nerve blocks (sPNBs) represent promising candidates for controlling postoperative pain, reducing dependence on opioid medications, and reducing postoperative constipation and ileus. However, there is not yet complete consensus regarding their efficacy. The primary aim of this study was to assess the impact of various sPNBs on patient short-term opioid demands and pain management parameters.
Methods: This single-center study retrospectively reviewed a cohort of 94 adult, elective surgery inpatients (ASA physical status I-III) scheduled for different operations. Sixty-four (68.1%) were selected for sPNB administration (group 1) and compared to the untreated group (group 0) for different clinical parameters.
Results: Contrary to the starting hypothesis, a higher proportion of group 1 patients experienced increasing pain intensities during the immediate postoperative period (P < 0.05, Fisher’s exact test), while requiring more bowel care medications (P < 0.05, χ2 test). Multiple linear regression modeling, however, showed that recovery time positively correlated with the opioid amount consumed (P < 0.01). Although limited, the results obtained in this study do not support an analgesic efficacy for sPNBs.
Conclusion: In conclusion, even though our data must be viewed within the limitations of our retrospective study and small group size, we did not find any compelling evidence for the efficacy of sPNB administration in the perioperative period.
Keywords: Single-shot peripheral nerve block; Opioid; Pain management; Postoperative recovery
| Introduction | ▴Top |
Postoperative recovery is a critical issue in healthcare settings worldwide. Pain management is a key component of this recovery. Adequate pain control improves ambulation, decreases hospitalization costs, lowers the risk of chronic post-surgical pain and persistent opioid use, and improves patient satisfaction [1-8]. Many everyday surgical operations labeled “minor” provoke acute postoperative pain [9]. The relevance of pain appraisal is underscored by the fact that roughly 100 million medical treatments are carried out in the United States annually, half of which are surgeries [10-12]. More concerningly, these numbers are projected to inflate in the near future [10, 12, 13], as orthopedic interventions have already increased steeply in the last decades [14]. Assessing pain intensity is moreover necessary because it is positively correlated with increasing numbers of postoperative impairments [8, 15-17].
Despite this, a substantial number of surgical patients experience insufficient perioperative pain relief [17-19]. Polls conducted in the United States (1993, 2003, and 2012) showed that poor postoperative pain control has persisted in severity and diffusion across three decades. In recent studies, 80-86% of patients reported being afflicted by postoperative pain, and 75-88% of this group experienced moderate to extreme pain intensities [2, 20]. Outside the perioperative period, these issues are paralleled by high incidences of persistent postsurgical pain (PPP), which affects 30-50% of patients and thus represents a significant societal toll [8, 21, 22]. The formulation of the current guidelines for pain management contributes to the ongoing undertreatment of postoperative pain. Often, these instructions are deficient in evidence [10], provide recommendations that are too general to be applied on specific cases [4], or lack unanimous consensus by different institutions [23, 24].
Clinical pain control has nonetheless progressed remarkably in the last few decades, leading to the development of new drugs, advancements in techniques, and innovative guidelines and protocols [1, 4, 25-28]. Even if multimodal analgesia remains vastly underutilized and insufficiently studied [29, 30], this strategy is considered the gold standard for ameliorating pain perceived by patients [1, 2, 29, 31, 32], particularly in postoperative settings [2]. This strategy utilizes the application of multiple different medications and procedures whose additive and synergistic interactions result in a more beneficial outcome when compared to the outcomes of monotypical treatments. With multimodal analgesia, the required dose of each drug used is lower, and the risk of adverse effects is minimized [1, 28, 29]. Multimodal therapies are cost-efficient, yielding higher patient satisfaction with lower side effects, and may lower the incidence of chronic postsurgical pain compared to opioids alone [2, 30].
In contrast to other analgesic medications, opioid drugs have a narrow therapeutic window. They have numerous detrimental effects, including phenomena of hyperalgesia, tolerance development, and rehospitalization [2, 29, 33-36]. Furthermore, their over-prescription has spurred an epidemic of opioid dependency in North America [2, 3, 20, 37]. Despite the adverse effects, however, these substances are still routinely administered intraoperatively as potent analgesics acting in the postoperative phase, and continue to represent a cornerstone of postoperative pain management [2, 4, 29, 34-37]. Fortunately, comprehensive clinical recommendations and novel protocols have recently been developed to minimize the risks associated with the injudicious use of opioids [38, 39]. Nevertheless, discarding opioids altogether is currently unfeasible [40], because the efficacy of opioid-free anesthesia (OFA) remains relatively untested and controversial to date [2, 36, 41-43]. Additionally, modern OFA regimens cannot provide analgesia throughout the entire perioperative period [44], and some nonopioid medications pose safety risks of their own [4, 43, 45]. Nonetheless, opioid-related complications have spurred a growing interest in opioid-sparing treatments [29, 43]. These therapies are ideally intended to replace the clinical use of opioids in time [36].
Considering the multimodal anesthesia paradigm, there is a wealth of medications and adjuvants that can be employed for opioid-free pain control [2, 29]. Among these, regional anesthesia driven by nerve blocks (peripheral and neuraxial blocks) appears to be a promising candidate for perioperative pain management [46, 47]. In many surgical specialties, peripheral nerve blocks have been increasingly adopted. However, both peripheral nerve blocks and neuraxial blocks remain reported as highly underutilized [14, 30, 47-49].
Peripheral nerve blocks are widely used on disparate body parts, are less invasive than neuraxial techniques, and have yielded successful results for many interventions in which other practices have been less effective [2, 49-51]. However, their usage by physicians has remained relatively low, with most anesthesiologists reporting performing less than five per month [52, 53]. Single-shot peripheral nerve blocks (sPNBs) involve the administration of a one-time dose of local anesthetic. They allow for pain relief with minimal invasion. The potent local analgesia conveyed by these drugs has a variable duration (< 1 h to > 1 day), depending on the pharmacology of the block used, its concentration, the injection’s area, and the patient’s response [50, 54, 55].
Although opioid consumption seems to be decreased in most cases, research has not yet established the exact relationship between nerve block administration and opioid consumption for postoperative pain management [49]. Considering an improvement in health care quality, the primary aim of this study was to investigate retrospectively how different sPNBs impact the opioid consumption of patients undergoing different surgical operations. In this study, it is hypothesized that sPNBs will decrease opioid consumption while improving patient satisfaction in the immediate postoperative period.
| Materials and Methods | ▴Top |
This study has been reviewed by the IRB at Marchand Institute and was found to be exempt from IRB review (November 2021). Data used were exempt from consent to participate or publish secondary to the nature of the study being a retrospective cohort, retrospectively looking at collected data. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. This study did not involve the use of any animals.
This single-center study reviewed retrospectively a cohort of 94 (33 males and 61 females, 35.1% and 64.9% of the total, respectively) adult, elective surgery inpatients (ASA physical status I-III), aged 32 - 91 years of age, scheduled for different operations. All surgical patients during this time period were recorded. The time period was preset secondary to the availability of the staff to volunteer for survey completion and data collection. The population examined did not include intubated, emergent, or intensive care unit (ICU) patients. Of the patients, 96.8% (91 of 94) were operated under general anesthesia. Date and time of discharge from the post-anesthesia care unit (PACU) and hospital were recorded. The discharge date from the PACU ranged from February 25, 2021 to May 5, 2021. The discharge date from the hospital ranged from March 10, 2021 to May 7, 2021. Surveys were taken shortly after discharge from PACU, with dates ranging from February 26, 2021 to May 6, 2021. The “all-comers” approach adopted by our researchers for this study was agreed to approximate the surgical volume and variety of the local community. The variety of surgical operations reported in the present study would have hindered an informative statistical analysis, therefore, the surgeries were grouped into homogeneous categories according to the German procedure classification (OPS) and the scheme used by Gerbershagen et al (2013) [17] to allow for comparisons.
The quantity of opioids taken orally for postoperative analgesia was recorded to assess the quality of pain control. The opioid doses were delivered via routine administration as described in our clinical protocols as well as via patient-controlled analgesia (PCA) pump. The opioid dosages were converted to an opioid oral morphine milligram equivalent (MME) using a standard opiate agent conversion chart to allow for a comparison of the different drugs administered [56]. All opioid doses administered throughout the 4 days following hospital discharge (postoperative days 0 - 3) were recorded, even those not explicitly requested by patients but dispensed as part of our protocol. No medical adjuncts were used in this study. Sixty-four of 94 patients (68.1%) were selected for the administration of a sPNB, and eight of these (8.5%) received two different blocks. The adductor canal (AC) block was the most administered (46.9%), whereas the second-most delivered block, the erector spinae plane (ESP), involved only 11% of the patients. Subsequently, to further appraise the quality of care provided, the following questionnaire was filled out by each patient: 1) What would you rate your average pain score on a 0 - 10 scale (10 = intense) for the first 12 h after surgery? When did it start? 2) Has your pain score changed at any point in the postoperative phase? Has it increased or decreased? What makes it worse or better? 3) Have you required pain medication since surgery? If so, how often? 4) On a 1 - 10 (10 = extremely satisfied) scale, how would you rate the pain management provided to you after surgery? 5) Did you experience postoperative nausea and/or vomiting (PONV)?
The statistical analyses were performed with the R software version 4.0 (R Core Team, 2021) [56] and the Jamovi software version 1.8 (The Jamovi Project, 2021) [57]. To test the efficacy of nerve blocks as analgesic medication on the treated versus untreated group (group 1 and 0, respectively; group 1 also included the patients treated with two nerve blocks), the Mann-Whitney U-test was performed for quantitative variables, while the χ2 or Fisher’s exact test was carried out for qualitative variables. The absence of inter-group differences was considered the null hypothesis (P < 0.05). For statistical convenience, all patients were considered operated under general anesthesia. The following parameters were treated as quantitative variables: “total consumption of opioids (MME)” (equivalent to the sum of all the opioid doses consumed during the four postoperative days), “pain rate reported (1 - 10)”, “satisfaction with pain management (1 - 10)”, “pain onset time (h)” (expressed in postoperative elapsed hours), and “age”. On the other hand, the following parameters were considered qualitative variables: “progress of pain perception” (increased, decreased, or constant), “opioids requested” (yes, no), “opioids received” (yes, no), “bowel care received” (yes, no), and “PONV” (yes, no).
Furthermore, a multiple linear regression model was built to explore potential relationships between the variables. “Total consumption of opioids (MME)” was set as the dependent variable, while the remaining parameters were used as independent variables. Qualitative variables were included by setting one of the possible states of each as the reference level (e.g., “request of opioids (no)” constituted the reference level of “request of opioids (yes)”). Parameters considered independent variables but that were not tested in the previous part of the analysis included “bowel activity (yes)”, “nerve block (1)”, “nerve block (2)” (in this case, administration of two nerve blocks was initially maintained as a separate category; “nerve block (0)” constituted the reference level of both), “recovery time (h)” (the intervening time between PACU and hospital discharge), and “sex”. The null hypothesis expected the absence of any significant regressor among the independent variables selected (P < 0.01). However, the regressors can act as confounders of each other, resulting in noise due to multicollinearity. To account for this issue, a model selection procedure was computed to obtain a second optimal model. This process consisted of using the stepwise backward elimination, stepwise forward selection, and best subset selection methods (implemented with the AIC criterion). The best subset selection method generated the optimal model with relatively any number of variables, and from it, the leave-one-out cross-validation (LOOCV) yielded the model with the optimal number of regressors.
| Results | ▴Top |
Cumulative postoperative opioid consumption (“total consumption of opioids (MME)”) was virtually equivalent (P = 0.483, Mann-Whitney U-test) between group 0 and 1. Therefore, we cannot reject the starting null hypothesis (Fig. 1a). A similar equivalence was recovered with the between-group comparisons in regards to “pain rate reported (1 - 10)” (P = 0.716, Mann-Whitney U-test), and “satisfaction with pain management (1 - 10)” (P = 0.473, Mann-Whitney U-test) (Fig. 1b-d). Conversely, the between-group comparisons concerning “progress of pain perception” and “bowel care received” yielded significant differences (P = 0.011, Fisher’s exact test, and P < 0.001, χ2 test, respectively) (Fig. 2). The between-group comparison for the qualitative variables “request of opioids” and “opioids received” did not show any significant discrepancy (P = 0.332 and P = 0.442, Fisher’s exact test, respectively). Additionally, the Fisher’s exact tests performed on each established surgery category (type and respective percentage of total surgeries are the following: “orthopedics, trauma”, 62.8%; “general surgery”, 22.3%; “neurosurgery”, 9.6%; “urology”, 3.2%; “gynecology”, 2.1%) for the inter-group comparison were not significant. Therefore, these variables were excluded from the multiple linear regression analysis to reduce statistical noise.
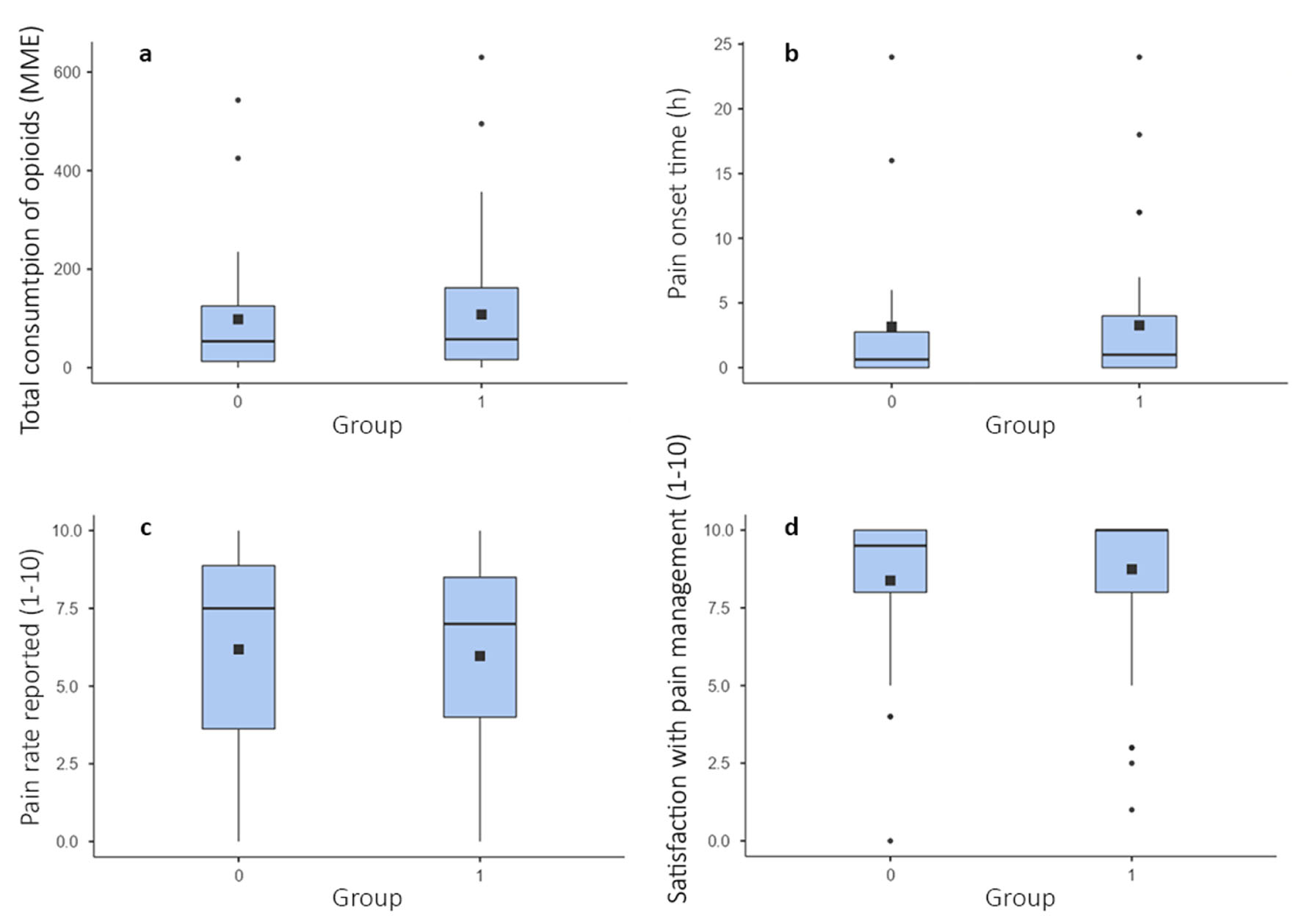 Click for large image | Figure 1. Box plots illustrating the results of the Mann-Whitney U-tests performed on four relevant quantitative variables for the between-group comparison: (a) total consumption of opioids (MME), (b) pain onset time, (c) pain rate reported (1 - 10), and (d) satisfaction with pain management (1 - 10). All tests are non-significant. In-box partitions represent means. Square dots represent medians. Box edges represent 25th and 75th percentiles. Whiskers represent 5th and 95th percentiles. Round dots represent outlying values. P < 0.05. |
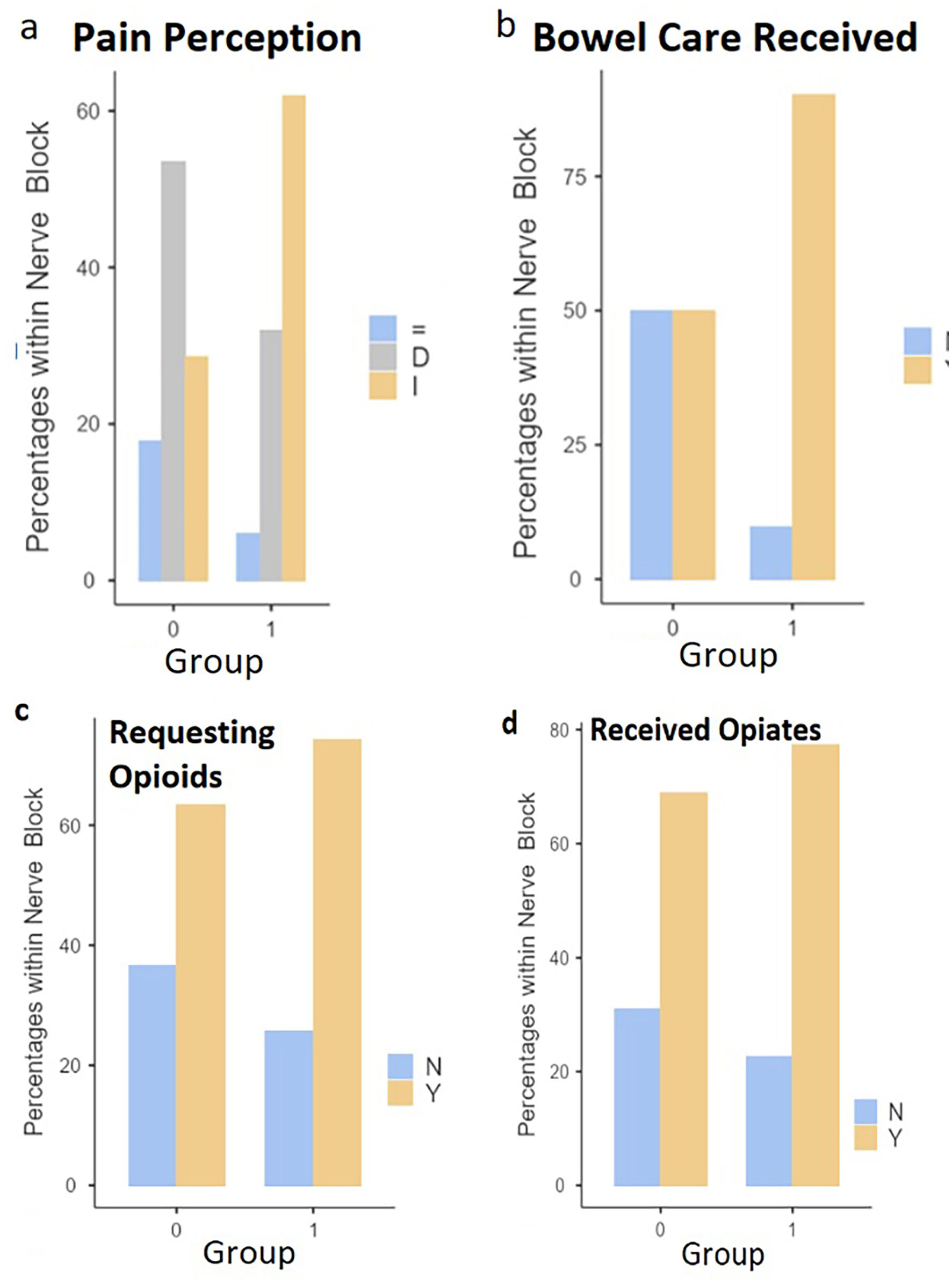 Click for large image | Figure 2. Bar plots illustrating the between-group comparisons concerning (a) pain perception, (b) bowel care received, (c) requesting opioids, and (d) received opioids variables. I: increased; D: decreased; =: constant (P = 0.011, Fisher’s exact test); Y: yes, N: no (P < 0.001, χ2 test). P < 0.05. |
The complete multiple linear regression model (P = 0.0003325, F-statistic test) evaluated the variable “recovery time (h)” as the sole significant regressor relative to “total consumption of opioids (MME)” (Table 1, P < 0.01). Thirty-three observations were eliminated due to missing values. All model selection methods resulted in a refined model (P = 2.79 × 10-6, F-statistic test) with three regressors: “age”, “nerve block (1)”, and “recovery time (h)” (Table 2, P < 0.01). “Nerve block (2)” was initially included in the refined model, although it was successively included in “nerve block (1)” due to the paucity of observations rendering this separate variable non-significant. In the refined model, “recovery time (h)” was again maintained as the only variable significantly correlated with “total consumption of opioids (MME)” (P = 7.81 × 10-7).
 Click to view | Table 1. Complete Multiple Linear Regression Model With “Total Consumption of Opioids (MME)” as Regress (P = 0.0003325, F-statistic test) |
 Click to view | Table 2. Refined Multiple Linear Regression Model With “Total Consumption of Opioids (MME)” as Regress (P = 2.79 × 10-6, F-statistic test) |
| Discussion | ▴Top |
As the results show, group 0 and 1 only differ for one of the clinical parameters relevant to the working hypothesis. In Figure 2a, it is evident that a higher proportion of group 1 patients experienced increasing pain intensities throughout the postoperative period compared to group 0 patients, and vice versa for decreasing pain intensities. Therefore, the part of the starting hypothesis expecting an ameliorated condition in patients treated with sPNBs must be rejected and revised. Also, it is to be concluded that the administration of sPNBs did not reduce nor affect opioid consumption, and the corresponding prediction in the starting hypothesis is to be rejected. Comparing the means and medians in Figure 1a renders it possible to appreciate a heightened opioid consumption within group 1, although this is insignificant. As a secondary outcome, it is noteworthy that group 1 patients also required substantially more bowel care medications (Fig. 2b). To our knowledge, a correlation between sPNBs and the need for bowel care has not been reported elsewhere. Furthermore, the multiple linear regression analysis was uninformative considering the working hypothesis (Tables 1 and 2). Although the “nerve block (1)” variable is included in the refined model (Table 2), it is insignificantly correlated with “total consumption of opioids (MME)” (P < 0.01). Even if P < 0.05 is considered, the significance level of “nerve block (1)” is not sufficiently low enough to infer a robust correlation. Additionally, the relative estimate contradicts the starting hypothesis. As the only highly significant variable, “recovery time (h)” is uninformative for this study since opioid reliance is known to be correlated with prolonged hospitalization (section “Introduction”).
The results of the present study were unexpected, because much evidence has been gathered on the efficacy of peripheral nerve blocks for postoperative pain relief while avoiding the deleterious side effects provoked by other systemic medications [2, 49-51]. However, high-quality evidence is scarce. Competent studies have shown continuous peripheral nerve blocks (cPNBs) to be superior to sPNBs in terms of therapeutic window, particularly for cases of severe orthopedic interventions, like knee arthroplasty. Concomitantly, the efficacy of nerve blocks in alleviating pain is mirrored by a reduced need to consume opioids [31, 49, 58-65]. More specifically, these assertions have been verified for the analgesic power of AC [66-70], femoral [71, 72], femoral triangle [70], ESP [73], transverse abdominis plane (TAP) [74, 75], and other nerve blocks. Despite exerting a protracted analgesic effect compared to sPNBs, cPNBs have been restricted to inpatient settings for safety purposes secondary to the possible complications arising from the use of catheters [49]. Moreover, the related trials have mostly revolved around a few specific surgeries (e.g., knee arthroplasty), and many studies reported contrasting results.
For instance, the prospective, randomized trial of Elkassabany et al (2019) [76] that examined the efficacy of continuous versus single-shot AC blocks found no clinically meaningful differences, except for some marginal advantages provided by the continuous block.
Additionally, the three study groups displayed a similar opioid consumption rate, albeit this outcome did not represent the primary goal of the overall trial. A comparable investigation carried out by Lee et al (2018) [77] inferred a similar overall conclusion. Correspondingly, the randomized, double-blinded trial by Dixit et al (2018) [78] concerning the comparison of single-shot and continuous femoral nerve blocks did not yield any significant difference between the two block types with respect to pain management, opioid consumption, and other clinical parameters. In contrast with part of these findings, the recent meta-analysis by Ma et al (2020) [79] revealed the association between continuous femoral blocks and a decreased need for opioids in the immediate postoperative period. The pain scores, incidence of nausea, and duration of hospitalization were insignificantly different. In opposition to this interpretation, a similar systematic study by Li et al (2020) [80] inferred a substantial superior analgesic potency of the continuous femoral nerve block compared to its single-shot counterpart. Dissimilar conclusions like those mentioned above might be generated by the different parameters associated with statistical clinical relevance or the different methodologies used. Some discrepancies highlighted between the utilization of the two block typologies help to circumscribe their ideal hospital setting. The double-blinded, randomized, controlled trial conducted by Turner et al (2018) [81] also retrieved an equivalence between the two types of AC blocks, despite the continuous one proving to be more beneficial after 36 - 42 h from its application. As secondary appraisals, no discrepancies concerning opioid consumption, patient satisfaction, or incidence of PONV were noted. These considerations warrant further clinical trials to assess the differences between cPNBs and sPNBs. Studies like the above mentioned add to the body of knowledge pointing to sPNBs as suitable candidates for early dismissal fast track surgical operations.
Some studies even discarded the existence of improvements provided by nerve blocks versus placebo. Jaeer et al (2012) [82] performed a double-blinded, randomized trial whereby a catheter was applied in the AC of all patients undergoing total knee arthroplasty during general anesthesia. However, only a portion of the group received the block, whereas the remainder received a sham injection. This study inferred decreased pain during knee flexion in the group treated with the block, while pain at rest and morphine consumption did not differ. Similarly, Goytizolo et al (2019) [83] regarded opioid consumption to be unaffected by administering the AC block as part of a multimodal regimen. Some studies even refused the use of PNBs as a viable alternative to other analgesic therapies. In the study conducted by Patel et al (2020) [84], a database of more than 300,000 patients subjected to primary total knee arthroplasty were queried to test the efficacy of both continuous and single-shot femoral blocks for postoperative pain control. Concerningly, patients treated with either type of block experienced more postoperative complications in comparison to the untreated sample. These adverse outcomes affected particularly the group using the continuous block, and were represented by a higher incidence of postoperative falls, inpatient readmission, and several systemic adverse conditions. Despite inaccurately counting the opioid doses consumed, the authors argued for an overall equivalent opioid consumption between all three study groups. Given its design, this study presents some limitations, such as the impossibility of accounting for adjuvants’ potential administration. Similarly, Lyngeraa et al (2019) [85] observed a negligible effect from both typologies of nerve blocks on opioid consumption decrease, although other parameters improved.
How sPNBs alone impact opioid consumption has been investigated by an insufficient number of studies (e.g., [86]). Abdallah et al (2015) [87] addressed this particular gap in their meta-analysis examining the efficacy of the single-shot interscalene block for successfully attenuating pain following shoulder surgery. Their study recovered a tangible short-lasting analgesic effect delivered by the block, accompanied by decreased postoperative opioid consumption, reduced PONV, and swifter recovery with early discharge. Instead, Seelam et al (2020) [86] assessed the analgesic efficacy of single-shot ultrasound-guided ESP block on patients undergoing mastectomies. The authors observed a reduced opioid consumption in the patient group treated with the block, although the incidence of PONV was equal across the whole study population. Conversely, Meftah et al (2020) [88] failed to detect a significant effect of sPNBs on opioid consumption compared to other analgesic treatments (e.g., periarticular injection). On the same side, Perlas et al (2013) [89] suggested implementing single-shot AC blocks as rescue analgesics.
The present study may add slightly to the body of knowledge expressing skepticism on using sPNBs over other analgesics. However, the conclusions reached in this work are far from firm. Several limitations and different methodologies hinder comparison with other clinical evidence. For instance, the “recovery time (h)” variable was defined based on the available time point data, and the distinction between pain on movement and at rest was not considered [12]. Furthermore, our investigation did not use a randomized, double-blinded, placebo-controlled approach. Missing values influenced the purity of the statistical analysis, whose low resolution was also influenced by the overabundance of categories compared to the small sample size. The insignificance relative to most of our parameters represented a strong liability because factors such as preoperative pain and sex are known to affect postoperative pain [12]. Additionally, the refined regression model was fitted to the data on which the model selection was performed, and this most likely rendered the inference estimates biased and distorted. Considering the plethora of often unaccounted-for clinical parameters and individual differences among patients [4, 14, 20], future clinical trials should correct all these shortcomings by focusing on a larger population and by including a restricted category number per parameter.
Acknowledgments
The Marchand Institute for Minimally Invasive Surgery would like to acknowledge the efforts of all of the students, researchers, residents and fellows at the institute who put their time and effort into these projects without compensation, only for the betterment of women’s health. We firmly assure them that the future of medicine belongs to them.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Data used were exempt from consent to participate or publish secondary to the nature of the study being a retrospective cohort, retrospectively looking at collected data. No identifiable patient data was used.
Author Contributions
The study was designed by JS, NS, SB and CR. The data were collected by CR and GM. The initial draft was written by SB, CR and GM. The calculations, data analysis, tables and figures were done by JS, SB, CR, and GM. Final draft and discussion were written by JS, NS, SB, and CR. All authors attest to significant contributions to this work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131-157.
doi pubmed - Ye H, Chen R, Lian X, Huang J, Mao Y, Lu R, Ai S, et al. Risk factors associated with postoperative pain and discomfort in oculoplastic surgery with general anesthesia: a prospective study. J Pain Res. 2018;11:407-415.
doi pubmed - Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546.
doi - Joshi GP, Kehlet H. Postoperative pain management in the era of ERAS: An overview. Best Pract Res Clin Anaesthesiol. 2019;33(3):259-267.
doi pubmed - Kurteva S, Iyabo OM, Karina G. General considerations for regional anesthesia practice. Essentials of Regional Anesthesia. Springer, Cham. 2018:3-19.
doi - Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292-298.
doi pubmed - Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015;62(2):203-218.
doi pubmed - Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377(9784):2215-2225.
doi - Cozowicz C, Poeran J, Memtsoudis SG. Epidemiology, trends, and disparities in regional anaesthesia for orthopaedic surgery. Br J Anaesth. 2015;115(Suppl 2):ii57-67.
doi pubmed - Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017;102:1-15.
- Meara JG, Leather AJ, Hagander L, Alkire BC, Alonso N, Ameh EA, Bickler SW, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569-624.
doi - Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273-e283.
doi pubmed - Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33(3):160-171.
doi pubmed - Vetter TR, Mascha EJ. Bias, confounding, and interaction: lions and tigers, and bears, oh my! Anesth Analg. 2017;125(3):1042-1048.
doi pubmed - Fletcher D, Stamer UM, Pogatzki-Zahn E, Zaslansky R, Tanase NV, Perruchoud C, Kranke P, et al. Chronic postsurgical pain in Europe: An observational study. Eur J Anaesthesiol. 2015;32(10):725-734.
doi pubmed - van Boekel RLM, Warle MC, Nielen RGC, Vissers KCP, van der Sande R, Bronkhorst EM, Lerou JGC, et al. Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 2019;269(5):856-865.
doi pubmed - Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934-944.
doi pubmed - Shanthanna H, Ladha KS, Kehlet H, Joshi GP. Perioperative opioid administration. Anesthesiology. 2021;134(4):645-659.
doi pubmed - Johansen A, Schirmer H, Stubhaug A, Nielsen CS. Persistent post-surgical pain and experimental pain sensitivity in the Tromso study: comorbid pain matters. Pain. 2014;155(2):341-348.
doi pubmed - Kehlet H, Jensen TS, Woolf CJ. The mechanism of post-thoracotomy pain syndrome (PTPS). Lancet. 2006;367:1618-1625.
doi - Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77-86.
doi pubmed - Kehlet H, Memtsoudis SG. Perioperative care guidelines: conflicts and controversies. Br J Surg. 2020;107(10):1243-1244.
doi pubmed - Mariano ER, Dickerson DM, Szokol JW, Harned M, Mueller JT, Philip BK, Baratta JL, et al. A multisociety organizational consensus process to define guiding principles for acute perioperative pain management. Reg Anesth Pain Med. 2022;47(2):118-127.
doi pubmed - Joshi GP. Enhanced recovery pathways for ambulatory surgery. Curr Opin Anaesthesiol. 2020;33(6):711-717.
doi pubmed - Peng LH, Min S, Jin JY, Wang WJ. Stratified pain management counseling and implementation improving patient satisfaction: a prospective, pilot study. Chin Med J (Engl). 2019;132(23):2812-2819.
doi pubmed - Prabhakar A, Mancuso KF, Owen CP, Lissauer J, Merritt CK, Urman RD, Kaye AD. Perioperative analgesia outcomes and strategies. Best Pract Res Clin Anaesthesiol. 2014;28(2):105-115.
doi pubmed - American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
doi pubmed - Ladha KS, Patorno E, Huybrechts KF, Liu J, Rathmell JP, Bateman BT. Variations in the use of perioperative multimodal analgesic therapy. Anesthesiology. 2016;124(4):837-845.
doi pubmed - Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149-160.
doi pubmed - Chen J, Zhou C, Ma C, Sun G, Yuan L, Hei Z, Guo C, et al. Which is the best analgesia treatment for total knee arthroplasty: Adductor canal block, periarticular infiltration, or liposomal bupivacaine? A network meta-analysis. J Clin Anesth. 2021;68:110098.
doi pubmed - Zech DFJ, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63(1):65-76.
doi - Rogers D. A system-level approach to reducing physician propagation of the opioid crisis: a brief history of increased opioid prescribing in the US and a review of expedited recovery after surgery protocol effects on opioid prescribing. Diss University of Pittsburgh. 2020.
- Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology. 2016;124(2):483-488.
doi pubmed - Lavand'homme P, Steyaert A. Opioid-free anesthesia opioid side effects: Tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. 2017;31(4):487-498.
doi pubmed - Lavand'homme P, Estebe JP. Opioid-free anesthesia: a different regard to anesthesia practice. Curr Opin Anaesthesiol. 2018;31(5):556-561.
doi pubmed - Earp BE, Silver JA, Mora AN, Blazar PE. Implementing a postoperative opioid-prescribing protocol significantly reduces the total morphine milligram equivalents prescribed. J Bone Joint Surg Am. 2018;100(19):1698-1703.
doi pubmed - Long DR, Lihn AL, Friedrich S, Scheffenbichler FT, Safavi KC, Burns SM, Schneider JC, et al. Association between intraoperative opioid administration and 30-day readmission: a pre-specified analysis of registry data from a healthcare network in New England. Br J Anaesth. 2018;120(5):1090-1102.
doi pubmed - Levy N, Quinlan J, El-Boghdadly K, Fawcett WJ, Agarwal V, Bastable RB, Cox FJ, et al. An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia. 2021;76(4):520-536.
doi pubmed - Brandal D, Keller MS, Lee C, Grogan T, Fujimoto Y, Gricourt Y, Yamada T, et al. Impact of enhanced recovery after surgery and opioid-free anesthesia on opioid prescriptions at discharge from the hospital: a historical-prospective study. Anesth Analg. 2017;125(5):1784-1792.
doi pubmed - Beloeil H. Opioid-free anesthesia. Best Pract Res Clin Anaesthesiol. 2019;33(3):353-360.
doi pubmed - Lavand'homme P. Opioid-free anaesthesia: Pro: damned if you don't use opioids during surgery. Eur J Anaesthesiol. 2019;36(4):247-249.
doi pubmed - Lirk P, Rathmell JP. Opioid-free anaesthesia: Con: it is too early to adopt opioid-free anaesthesia today. Eur J Anaesthesiol. 2019;36(4):250-254.
doi pubmed - Alexander JC, Patel B, Joshi GP. Perioperative use of opioids: Current controversies and concerns. Best Pract Res Clin Anaesthesiol. 2019;33(3):341-351.
doi pubmed - Chia PA, Cannesson M, Bui CCM. Opioid free anesthesia: feasible? Curr Opin Anaesthesiol. 2020;33(4):512-517.
doi pubmed - Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev. 2012;10:CD007105.
doi pubmed - Joshi GP, Kehlet H, Rawal N. Surgeon-administered regional analgesia to replace anaesthetist-administered regional analgesia: need for communication and collaboration. Br J Anaesth. 2019;123(6):707-709.
doi pubmed - Gabriel RA, Ilfeld BM. Use of Regional Anesthesia for Outpatient Surgery Within the United States: A Prevalence Study Using a Nationwide Database. Anesth Analg. 2018;126(6):2078-2084.
doi pubmed - Saporito A, Anselmi L, Sturini E, Borgeat A, Aguirre JA. Is outpatient continuous regional analgesia more effective and equally safe than single-shot peripheral nerve blocks after ambulatory orthopedic surgery? Minerva Anestesiol. 2017;83(9):972-981.
doi pubmed - Senapathi, Tjokorda Gde Agung, et al. Effectiveness of continuous adductor canal block versus continuous epidural analgesia in patients with total knee arthroplasty: A systematic review. Bali Journal of Anesthesiology. 2020;4(4):148.
doi - Kent C, et al. United States: complications associated with regional anesthesia (An American society of anesthesiologists' closed claims analysis). Complications of Regional Anesthesia. Springer, Cham. 2017:451-462.
doi - Rawal N. American Society of Regional Anesthesia and Pain Medicine 2010 Gaston Labat Lecture: Perineural catheter analgesia as a routine method after ambulatory surgery—effective but unrealistic. Reg Anesth Pain Med. 2012;37(1):72-78.
doi pubmed - Hadzic A, Vloka JD, Kuroda MM, Koorn R, Birnbach DJ. The practice of peripheral nerve blocks in the United States: a national survey [p2e comments]. Reg Anesth Pain Med. 1998;23(3):241-246.
doi pubmed - Helwani MA, Saied NN, Asaad B, Rasmussen S, Fingerman ME. The current role of ultrasound use in teaching regional anesthesia: a survey of residency programs in the United States. Pain Med. 2012;13(10):1342-1346.
doi pubmed - Spinal Epidural, Caudal Blocks. In: Butterworth IV JF, Mackey DC, Wasnick JD. eds. Morgan & Mikhail's Clinical Anesthesiology, 6e. McGraw Hill; 2018. Accessed December 28, 2021. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2444§ionid=193555666.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
doi pubmed - Team R, Core R. A language and environment for statistical computing. 2013:201.
- Sahin Murat Dogan, Eren Can Aybek. Jamovi: an easy to use statistical software for the social scientists. International Journal of Assessment Tools in Education. 2019;6(4):670-692.
doi - Aguirre J, Del Moral A, Cobo I, Borgeat A, Blumenthal S. The role of continuous peripheral nerve blocks. Anesthesiol Res Pract. 2012;2012:560879.
doi pubmed - Bingham AE, Fu R, Horn JL, Abrahams MS. Continuous peripheral nerve block compared with single-injection peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med. 2012;37(6):583-594.
doi pubmed - Borgeat A. Single-Shot Interscalene Block: Light and Shadows. Anesth Analg. 2015;120(5):995-996.
doi pubmed - Fredrickson MJ, Ball CM, Dalgleish AJ. Analgesic effectiveness of a continuous versus single-injection interscalene block for minor arthroscopic shoulder surgery. Reg Anesth Pain Med. 2010;35(1):28-33.
doi pubmed - Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113(4):904-925.
doi pubmed - Sun C, Zhang X, Song F, Zhao Z, Du R, Wu S, Ma Q, et al. Is continuous catheter adductor canal block better than single-shot canal adductor canal block in primary total knee arthroplasty? A GRADE analysis of the evidence through a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(20):e20320.
doi pubmed - Wang C, Chen Z, Ma X. Continuous adductor canal block is a better choice compared to single shot after primary total knee arthroplasty: A meta-analysis of randomized controlled trials. Int J Surg. 2019;72:16-24.
doi pubmed - Canbek U, Akgun U, Aydogan NH, Kilinc CY, Uysal AI. Continuous adductor canal block following total knee arthroplasty provides a better analgesia compared to single shot: A prospective randomized controlled trial. Acta Orthop Traumatol Turc. 2019;53(5):334-339.
doi pubmed - Kim MK, Moon HY, Ryu CG, Kang H, Lee HJ, Shin HY. The analgesic efficacy of the continuous adductor canal block compared to continuous intravenous fentanyl infusion with a single-shot adductor canal block in total knee arthroplasty: a randomized controlled trial. Korean J Pain. 2019;32(1):30-38.
doi pubmed - Li C, Xu H, Shen B, Yang J, Zhou Z, Kang P, Pei F. [Effect of continuous and single shot adductor canal blocks for postoperative analgesia and early rehabilitation after total knee arthroplasty]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(9):1049-1054.
- Shah NA, Jain NP, Panchal KA. Adductor canal blockade following total knee arthroplasty-continuous or single shot technique? role in postoperative analgesia, ambulation ability and early functional recovery: a randomized controlled trial. J Arthroplasty. 2015;30(8):1476-1481.
doi pubmed - Yu R, Wang H, Zhuo Y, Liu D, Wu C, Zhang Y. Continuous adductor canal block provides better performance after total knee arthroplasty compared with the single-shot adductor canal block?: An updated meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(43):e22762.
doi pubmed - Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102(4):1234-1239.
doi pubmed - Tangwiwat Suwimon. Comparison of single-injection and continuous femoral nerve block after primary total knee arthroplasty on postoperative pain control and length of hospital stay: a randomized, double-blind study. Thai Journal of Anesthesiology. 2021;47(4):337-347.
- Layera S, Aliste J, Bravo D, Saadawi M, Salinas FV, Tran Q. Motor-sparing nerve blocks for total knee replacement: A scoping review. J Clin Anesth. 2021;68:110076.
doi pubmed - Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: A pooled review of 242 cases. J Clin Anesth. 2019;53:29-34.
doi pubmed - Vonu PM, Campbell P, Prince N, Mast BA. Analgesic efficacy of nerve blocks after abdominoplasty: a systematic review. Aesthet Surg J. 2020;40(11):1208-1215.
doi pubmed - McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, Laffey JG. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2008;106(1):186-191.
doi pubmed - Elkassabany NM, Cai LF, Badiola I, Kase B, Liu J, Hughes C, Israelite CL, et al. A prospective randomized open-label study of single injection versus continuous adductor canal block for postoperative analgesia after total knee arthroplasty. Bone Joint J. 2019;101-B(3):340-347.
doi pubmed - Lee S, Rooban N, Vaghadia H, Sawka AN, Tang R. A randomized non-inferiority trial of adductor canal block for analgesia after total knee arthroplasty: single injection versus catheter technique. J Arthroplasty. 2018;33(4):1045-1051.
doi pubmed - Dixit V, Fathima S, Walsh SM, Seviciu A, Schwendt I, Spittler KH, Briggs D. Effectiveness of continuous versus single injection femoral nerve block for total knee arthroplasty: A double blinded, randomized trial. Knee. 2018;25(4):623-630.
doi pubmed - Ma HH, Chou TA, Tsai SW, Chen CF, Wu PK, Chen WM. The efficacy of continuous versus single-injection femoral nerve block in Total knee Arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2020;21(1):121.
doi pubmed - Li S, Zhou J, Li X, Teng X, Li Y, Du C, Zhu J. Analgesic impact of single-shot versus continuous femoral nerve block after total knee arthroplasty: a systematic review and meta-analysis. Adv Ther. 2020;37(2):671-685.
doi pubmed - Turner JD, Dobson SW, Henshaw DS, Edwards CJ, Weller RS, Reynolds JW, Russell GB, et al. Single-injection adductor canal block with multiple adjuvants provides equivalent analgesia when compared with continuous adductor canal blockade for primary total knee arthroplasty: a double-blinded, randomized, controlled, equivalency trial. J Arthroplasty. 2018;33(10):3160-3166.e3161.
doi pubmed - Jaeger P, Grevstad U, Henningsen MH, Gottschau B, Mathiesen O, Dahl JB. Effect of adductor-canal-blockade on established, severe post-operative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiol Scand. 2012;56(8):1013-1019.
doi pubmed - Goytizolo EA, Lin Y, Kim DH, Ranawat AS, Westrich GH, Mayman DJ, Su EP, et al. Addition of adductor canal block to periarticular injection for total knee replacement: a randomized trial. J Bone Joint Surg Am. 2019;101(9):812-820.
doi pubmed - Patel AH, Ross BJ, Ofa SA, Flick TR, Sanchez FL, Sherman WF. The impact of femoral nerve anesthesia on short-term clinical outcomes and opioid claims after total knee arthroplasty. Arthroplast Today. 2020;6(4):1016-1021.e1019.
doi pubmed - Lyngeraa TS, Jaeger P, Gottschau B, Graungaard B, Rossen-Jorgensen AM, Toftegaard I, Grevstad U. Comparison of the analgesic effect of an adductor canal block using a new suture-method catheter vs. standard perineural catheter vs. single-injection: a randomised, blinded, controlled study. Anaesthesia. 2019;74(11):1397-1405.
doi pubmed - Seelam S, Nair AS, Christopher A, Upputuri O, Naik V, Rayani BK. Efficacy of single-shot ultrasound-guided erector spinae plane block for postoperative analgesia after mastectomy: A randomized controlled study. Saudi J Anaesth. 2020;14(1):22-27.
doi pubmed - Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth Analg. 2015;120(5):1114-1129.
doi pubmed - Meftah M, Boenerjous-Abel S, Siddappa VH, Kirschenbaum IH. Efficacy of adductor canal block with liposomal bupivacaine: a randomized prospective clinical trial. Orthopedics. 2020;43(1):e47-e53.
doi - Perlas A, Kirkham KR, Billing R, Tse C, Brull R, Gandhi R, Chan VW. The impact of analgesic modality on early ambulation following total knee arthroplasty. Reg Anesth Pain Med. 2013;38(4):334-339.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


