| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 14, Number 4, April 2022, pages 147-157
State of the Art, Current Perspectives, and Controversies of Budd-Chiari Syndrome: A Review
Paschalis Gavriilidisa, c, Gabriele Marangonia, Jawad Ahmada, Daniel Azoulayb
aDepartment of HBP Surgery, University Hospitals of Coventry and Warwickshire NHS Trust, Clifford Bridge Road, Coventry CV2 2DX, UK
bDepartment of Hepato-Biliary and Liver Transplantation Surgery, Paul Brousse University Hospital, Paris-Saclay University, 94800 Villejuif, France
cCorresponding Author: Paschalis Gavriilidis, Department of HBP Surgery, University Hospitals Coventry and Warwickshire NHS Trust, Clifford Bridge Road, Coventry CV2 2DX, UK
Manuscript submitted April 12, 2022, accepted April 25, 2022, published online April 30, 2022
Short title: Budd-Chiari Syndrome
doi: https://doi.org/10.14740/jocmr4724
| Abstract | ▴Top |
Background: Budd-Chiari syndrome (BCS) is an eponym that includes a group of conditions characterized by partial or complete hepatic venous tract outflow obstruction, and the site of obstruction may involve one or more hepatic veins, inferior vena cava, or the right atrium. The classification of BCS is based on etiology, site of obstruction, and duration. Its etiology is very heterogeneous; in particular, hepatic vein thrombosis is the most common type of obstruction and myeloproliferative disorder, the most common thrombophilic disorder, in the West. In Asian countries, the type of obstruction, thrombophilic disorders, clinical features, and treatment strategies vary widely from region to region. Although the cause can be identified in 90% of patients with the help of gene mutation testing, BCS remains under-recognized in many countries. A higher prevalence of acute cases has been reported in the West than in the East. This global and regional heterogeneity raises several challenges regarding the evaluation, management strategy, and individualized approach of BCS. This study aimed to conduct a systematic review of BCS to elucidate treatment strategy options.
Methods: PubMed, Embase, Cochrane Library, and Google Scholar databases were searched systematically.
Results: Sixty-nine pertinent articles were retrieved and included in the present study.
Conclusions: Further research on the following three topics would help define individualized treatment strategies. The first is a better understanding of the molecular pathways underlying the thrombophilic conditions implicated in the pathogenesis of BCS. The second is the role of the genotype and gene mutations in the determination of coagulation status of patients with BCS. The third is the definition of clear criteria and development of a common prognostic index to risk stratify the patients at presentation and consequently detect candidates for invasive therapies.
Keywords: Budd-Chiari syndrome; Hepatic outflow obstruction; Hepatic vein thrombosis; Vascular liver diseases; Transjugular intrahepatic portosystemic shunt; Liver transplantation; Systematic review
| Introduction | ▴Top |
Budd-Chiari syndrome (BCS) is an eponym describing a heterogeneous group of complete or partial hepatic venous tract outflow obstruction conditions, regardless of the type of mechanism. Any complete or partial hepatic venous outflow obstruction leads to increased hepatic sinusoidal pressure which results in liver congestion and portal hypertension. This leads to hepatocyte hypoxia and dysfunction. If the condition remains under-recognized and the obstruction is not corrected in a timely manner, this can lead to hepatocyte necrosis, progressive centrilobular fibrosis, nodular regenerative hyperplasia, and ultimately cirrhosis [1-4]. Therefore, if the hepatic flow is restored either by development of the portal venous collateral system or by creation of a portosystemic shunt, the hepatic sinusoidal pressure will be reduced drastically [5]. Thus, treatment strategies should be based on detailed knowledge of the underlying etiological factors, clinical presentation, and baseline liver function.
| Materials and Methods | ▴Top |
Literature search strategy
A literature search was performed from inception until November 2021 in Medline (PubMed), Embase, Cochrane Library and Database for Systematic Reviews (CDSR), Google Scholar, and National Institute for Health and Clinical Excellence (NICE) databases using free text and MeSH terms (Budd-Chiari syndrome, hepatic outflow obstruction, hepatic vein (HV) thrombosis, vascular liver diseases, transjugular intrahepatic portosystemic shunt, liver transplantation (LT), and systematic review). References cited in the retrieved articles were manually checked for further analysis. Any disagreements between the authors were resolved by consensus.
Question
What is the current state of assessment, risk stratification, and medical and surgical treatment strategies for patients with BCS and what issues remain unsolved? What are the future research directions in the management of patients with BCS that may maximize the application of their individualised treatment?
| Results | ▴Top |
Sixty-nine pertinent articles were retrieved and included in the present study, including 32 reviews [1-4, 6-33], 32 retrospective [34-64], four prospective [65-68], and one experimental studies [5] (Fig. 1). Due to the large number of included reviews and great heterogeneity of retrospective and prospective studies, meta-analysis was not performed.
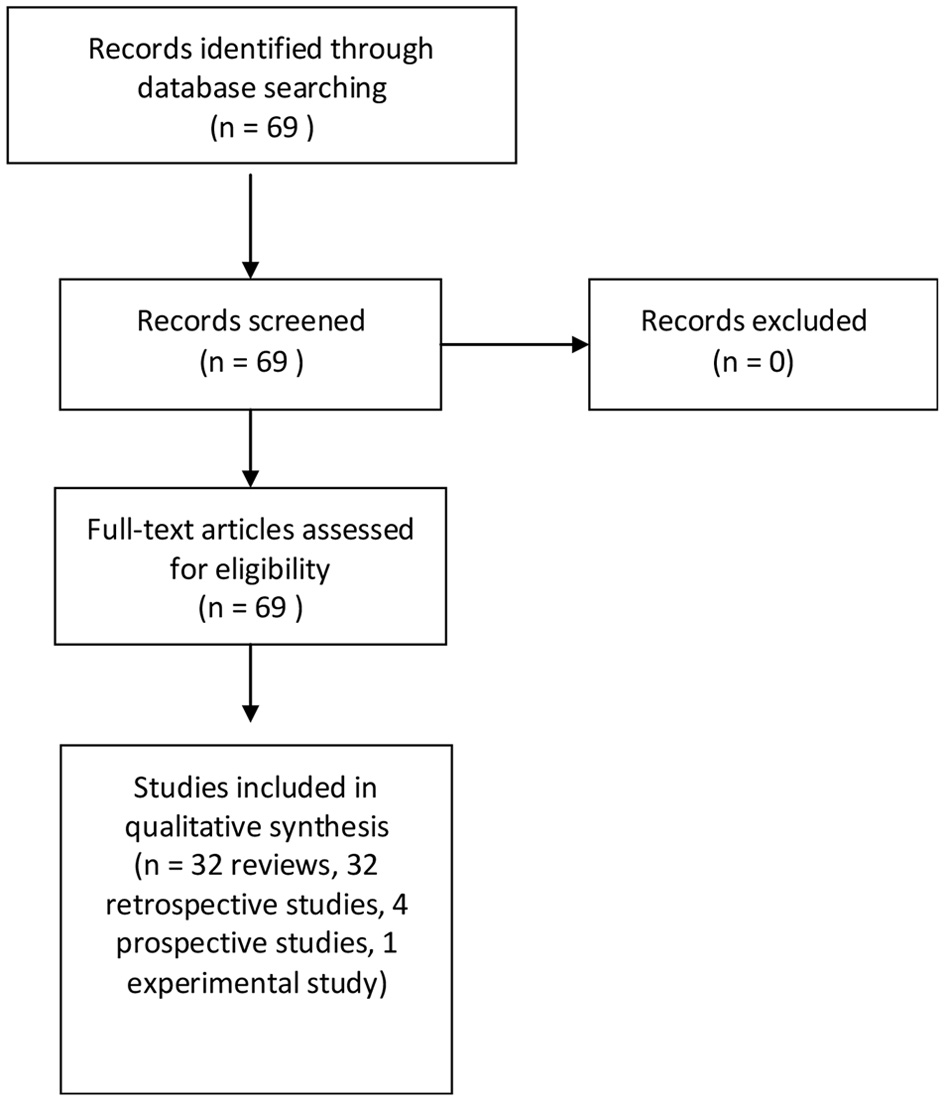 Click for large image | Figure 1. Diagram of the search strategy. |
| Discussion | ▴Top |
Characteristics of BCS
Epidemiology
In 2019, a meta-analysis on the epidemiology of BCS reported an incidence and prevalence rate of 0.168 - 4.09 and 2.40 - 33.10 per million, respectively. The absolute pooled incidence rate was lower in Asia than in Europe (0.469 - 2.0 per million). However, the above results should be treated cautiously because they were based on only two Asian and four European studies [14]. In the future, well-designed studies are required to detect precise epidemiological data.
Etiology
It has been reported that 75% of primary BCS cases are caused by prothrombotic conditions [7, 34]. These are divided into acquired and inherited types. The most common causes of acquired prothrombotic conditions of primary BCS are BCR-ABL-negative myeloproliferative neoplasms (MPNs), idiopathic myelofibrosis, and essential thrombocythemia. The factor V Leiden mutation, G20210A prothrombin gene mutation, and inherited protein S and C deficiencies are the most common inherited prothrombotic conditions of primary BCS. Of note, the incidence rate of MPNs is low among Chinese patients with BCS [7-9, 34].
It has been reported that 30-50% of patients with BCS are diagnosed with the JAK2 V617F mutation. Therefore, routine screening for JAK2 V617F may contribute to the early diagnosis of MPNs in BCS patients [10].
Membranous occlusion of the HV and/or inferior vena cava (IVC) is more common in Asian patients than in Western patients [11].
Membranous obstruction of the inferior vena cava (MOVC) predominantly affects the hepatic portion of IVC. The main cause is considered to be bacterial infection, and its prevalence is higher in developing countries than in the West. It has been reported that it is more frequently associated with hepatocellular carcinoma (HCC) than classic cases of BCS. Based on the above data, BCS and MOVC should be considered as two separate clinical entities and should be managed accordingly. Thus, a new disease concept has been proposed for MOVC “obliterative hepatocavopathy” [12-15]. There are two opposing theories regarding the pathogenesis of MOVC: congenital vascular malformation and thrombotic theory.
Recently, Shrestha et al suggested that MOVC is caused by bacteria-induced thrombophlebitis [14]. Mechanical disruption or stenting of the obstructed IVC and/or HVs may restore hepatic outflow, closely mimicking normal physiology [11].
It has been reported that 6-47% of women with BCS manifest during pregnancy. BCS has also been reported as a cause of primary infertility. Shukla et al, from Mumbai, India, reported that the prevalence rate of primary infertility was 25% in women with BCS and 6.3% in the general population. Of note, during pregnancy, heparin is preferred to warfarin because it does not cross the placenta [16, 35-37].
Clinical presentation
The clinical presentation of BCS varies widely from fulminant to asymptomatic presentations. The principal hallmark features of BCS are hepatomegaly, ascites, abdominal pain, and the presence of dilated superficial abdominal wall veins. Manifestation of clinical signs and features mainly depends on the degree of involvement of HVs and IVC. Fulminant presentation occurs when all three HVs are obstructed, and its incidence rate is very low [38].
There is a discrepancy in the prevalence rate between the West and East; acute presentation more commonly occurs in the West, whereas in the East, chronic presentation is more common, and its duration may expand from 6 months to 30 years. Furthermore, 15% of the cases are completely asymptomatic and diagnosed incidentally during the evaluation of chronic liver diseases or abnormal liver function tests (LFTs) [17, 39, 40]. In general, laboratory results are not specific; however, high protein concentrations may be detected in ascitic fluid. However, some adolescent patients may present with hepatomegaly without ascites; this is due to more efficient portal system decompression due to collateral formation secondary to potent angiogenic factors during adolescence [18]. Variceal bleeding may occur in 5-21% of cases and portal vein thrombosis in 15% of cases. Notably, some of the cases may regard the acute-on-chronic phenomenon which is secondary to the new thrombus superimposed on underlying chronic stenosis or obstruction. Pathologic examination can diagnose layers of thrombi of different ages. Acute chronic liver failure can be classified into three types: fulminant, acute, and subacute [19-21]. Therefore, although BCS has a low incidence rate, it should be considered in the differential diagnosis of any form of unexplained acute or chronic liver disease.
Classification
The classification of BCS is based on the following three pillars: etiology, level of obstruction, and duration of disease.
Based on etiology, BCS is classified into primary which includes intravenous thrombosis, webs, and endophlebitis, and secondary which includes tumors, parasitic cysts (echinococcosis), and abscesses that compress the venous system [11].
So far, there has been no common classification for use, and two classifications have been proposed based on the site of obstruction. Type I refers to obstruction of the IVC with or without secondary HV occlusion, whereas type II refers to the HV obstruction without IVC obstruction or compression. In addition, one contained three types and the other four types of BCS. Therefore, liver surgeons from centers of expertise should develop a single classification for better communication between the authors (Tables 1 and 2; Figs. 2 and 3) [3, 22, 41].
 Click to view | Table 1. Three Types of BCS Based on the Level of Obstruction |
 Click to view | Table 2. Four Types of BCS Based on the Level of Obstruction |
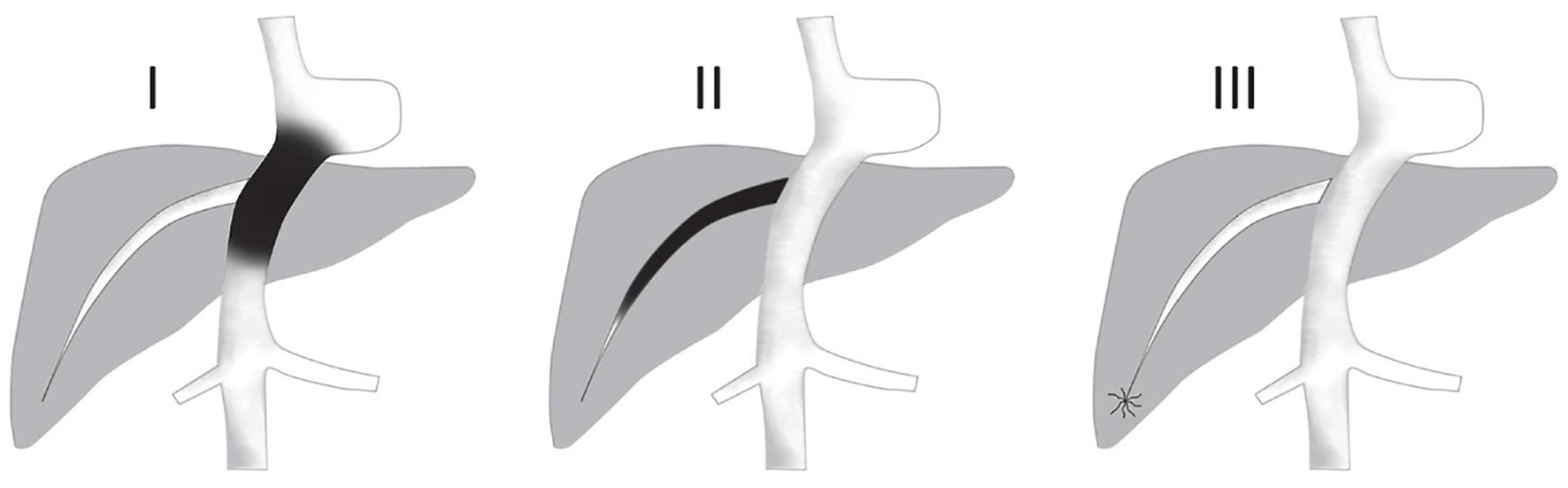 Click for large image | Figure 2. Types of Budd-Chiari syndrome according to levels of obstruction in Table 1. Type I: (Truncal) obstruction involves the IVC ± HVs. Type II: (Radicular) with obstruction at the level of HVs. Type III: (Veno-oclusive) obstruction at the level of small centrilobular veins. HV: hepatic vein; IVC: inferior vena cava. |
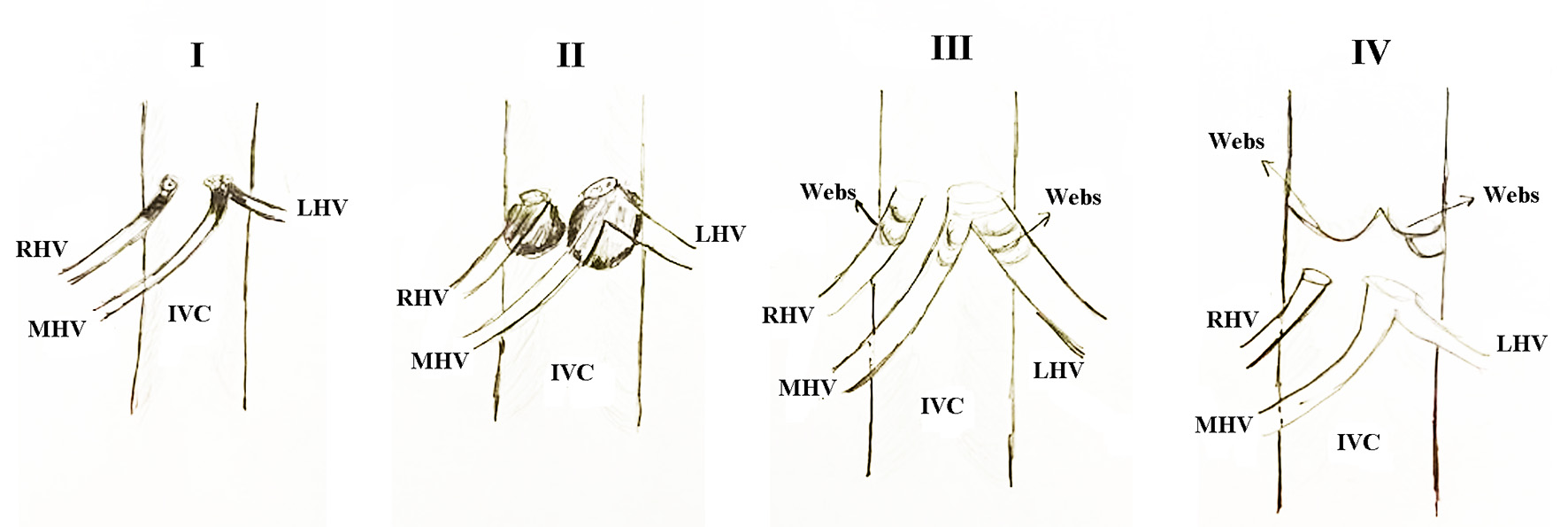 Click for large image | Figure 3. Types of Budd-Chiari syndrome according to levels of obstruction in Table 2. Type I: Hepatic vein obstruction or thrombosis without IVC obstruction or compression. Type II: Hepatic vein obstruction or thrombosis with IVC obstruction or thrombosis. Type III: Isolated hepatic venous webs. Type IV: Isolated IVC webs. RHV: right hepatic vein; MHV: middle hepatic vein; LHV: left hepatic vein; IVC: inferior vena cava. |
Based on the clinical features of onset and duration of disease, BCS is classified into fulminant, acute, subacute, and chronic (Table 3) [3, 22, 41]. In particular, fulminant is defined as any occurrence of hepatic encephalopathy within 8 weeks from the diagnosis of icterus. Acute is defined as any duration of less than 1 month with features of ascites and hepatic necrosis but without formation of venous collaterals. The subacute has an insidious onset and its duration lasts from 1 to 6 months with ascites, minimal hepatic necrosis, and development of portal and hepatic venous collaterals. Chronic is defined as any BCS that lasts for more than 6 months (Table 3) [22, 41]. Furthermore, acute-on-chronic liver failure (ACLF) can be classified into three types. In particular, type A was characterized by pathological findings of acute HV thrombosis or blockage of the stenting device. In type B, non-thrombotic acute episodes trigger an acute-on-chronic episode in a chronic BCS, and in type C, acute HV thrombosis triggers acute chronic episodes in non-vascular chronic liver disease (Table 4) [11].
 Click to view | Table 3. Classification of BCS According to the Duration of Disease |
 Click to view | Table 4. Types of Acute on Chronic BCS |
Prognostic indices for BCS
As with any other clinical entity similar to BCS, the need to quantify the disease severity and to identify patients at risk for disease progression, and increased morbidity and mortality make liver surgeons from the centers of excellence to develop prognostic indices. To date, there are five prognostic scores: the Clichy, New Clichy, Rotterdam, BCS-transjugular intrahepatic portosystemic shunt (TIPSS), and All India Institute of Medical Sciences Hepatic Venous Outflow Tract Obstruction (AIIMS-HVOVTO) scores (Table 5) [11, 42-45].
 Click to view | Table 5. Prognostic Indices for BCS |
The first prediction score was developed by the Clichy team to predict mortality in patients managed either medically or surgically with portosystemic shunts. They concluded that both treatments do not influence survival and have a poor prognosis in patients with scores > 5.4 [42]. Consequently, the same team further validated their prognostic index by adding a new parameter to pathological findings in the liver at the time of the first diagnosis. In particular, the morphological findings were classified into three types. They identified that type III, which included both chronic and acute morphological findings, simultaneously demonstrated significantly worse prognosis compared to patients with type I (acute HVOTO) and type II (chronic) BCS. Notably, both scores demonstrated similar prognostic accuracy [11].
Rotterdam score based on patients who underwent medical, TIPSS, or portosystemic shunts. This demonstrated that patients of class II who underwent surgical portosystemic shunts had improved survival compared to class I and II patients (Table 5) [43].
The BCS-TIPSS score was based on the results of 106 consecutive patients who underwent TIPSS and were followed up until orthotopic LT or demise. A BCS-TIPSS score > 7 is considered an indication for LT (Table 5) [43].
AIIMS-HVOTO was developed in 233 patients who underwent angioplasty/stenting as first-line treatment. Consequently, if the above treatment failed, TIPSS will be performed. The principal message of the above score is that even in patients with remarkably deranged LFTs, endovascular interventions essentially improve survival in patients with BCS (Table 5) [45].
Rautou et al reported that all prognostic indices can be used as valid predictors in clinical studies; however, these are insufficient tools for individualized treatment management of BCS patients [46].
Principles of management and outcomes of BCS
Prior to designing any treatment strategy, the following data are needed to design an individualized stepwise approach: in particular, the presence and size of intrahepatic venous collaterals; second, permeability status of the IVC; third, permeability status of intra- and extrahepatic portal veins; and fourth, data on the size of the caudate lobe vein and accessory HVs [23].
The treatment approach for BCS is stepwise and includes the following therapeutic options. First-line treatment is anticoagulation therapy; however, associated liver dysfunction coagulopathy makes coagulation function unpredictable in BCS patients. At the present is followed the treatment protocol as in deep venous thrombosis. It is prescribed low-molecular weight heparin (LMWH) for at least 5 - 7 days and life-long administration of warfarin, aiming at an international normalized ratio (INR) of 2-3. Only 15-20% of patients respond to medical therapy, and the rest are candidates for additional therapies such as catheter-directed thrombolysis, mechanical thrombectomy, angioplasty, stenting, creation of TIPSS, and LT [11, 24, 25] (Fig. 4).
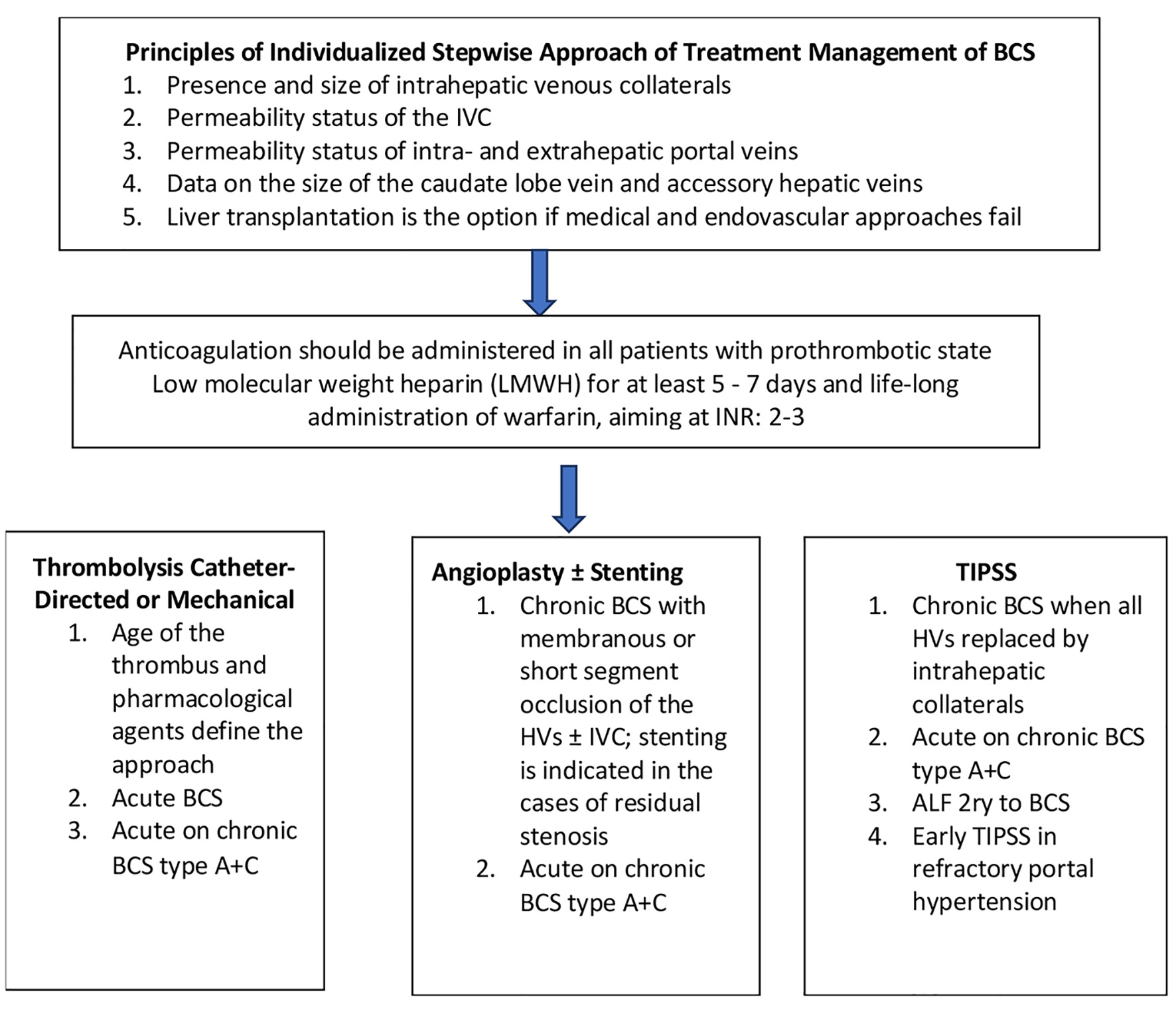 Click for large image | Figure 4. Diagram of stepwise therapeutic approach of BCS. BCS: Budd-Chiari syndrome. |
Acute BCS
In acute BCS, catheter-directed thrombolysis is the treatment of choice, but whether to proceed with pharmacological or mechanical thrombolysis depends on the age of the thrombus and the pharmacological agents used. In cases with hyperacute thrombi, pharmacological thrombolysis alone is effective [25].
There is scarce evidence regarding the use of TIPSS in patients with acute BCS. He et al reported that 37 patients with acute BCS underwent TIPSS with 100% technical success. There was a significant reduction in the portosystemic pressure gradient (PSPG), LFTs, and serum bilirubin levels [26]. In contrast, Mancuso et al reported a mortality rate of 50%. Four out of eight patients with acute BCS who underwent TIPSS died soon after the procedure [47].
Chronic BCS
Patients with chronic BCS are stratified mainly into two cohorts, and their treatment is tailored accordingly. One cohort included those with membranous or short segment occlusion of the HVs and/or IVC; the treatment of choice for them was angioplasty with or without stenting. Stenting is indicated in cases of residual stenosis following angioplasty. The second cohort included patients in whom all HVs were replaced by intrahepatic collaterals. In such cases, angioplasty is not suitable, and the treatment of choice is TIPSS [24, 25]. Doppler ultrasonography (US) and magnetic resonance (MR) venography were used to assess the response to intervention. Reductions of ascites and size of the caudate lobe are criteria for successful intervention [48, 65].
ACLF in BCS
ACLF is defined as any occurrence of hepatic disease manifesting with ascites, icterus, coagulopathy, and encephalopathy in patients with previously diagnosed or undiagnosed chronic liver disease or cirrhosis [27]. Based on the underlying liver disease and acute episode, ACLF can be classified into three types (Table 4) [11, 27]. Patients with types A and C are suitable for the treatment of thrombolysis or thrombectomy with stenting and TIPSS. Patients with type B ACLF should be treated as in any other ACLF. When the above interventions fail, LT is an alternative [49].
Acute liver failure (ALF) secondary to BCS
The prevalence of ALF was very low. Data based on two case series reported it for 0.9-15% of all cases of ALF [38, 50]. The US ALF study group reported 20 cases of ALF due to BCS in a total of 2,344 enrolled cases. The most common trigger factor was polycythaemia vera, and the majority were Caucasian females (84%) [50]. TIPSS is the first treatment option, with a reported survival of 50-80%. Simultaneously, the patients should be listed for emergency transplantation in cases of TIPSS failure [44].
Controversies in the management of BCS
Angioplasty alone vs. routine stenting
It has been reported that the incidence of restenosis after angioplasty recanalization is approximately 50% [28]. A recent randomized controlled trial (RCT) demonstrated that the cohort of patients with routine stenting had a significantly lower incidence rate of restenosis and symptom recurrence rate compared to a cohort of angioplasty alone [66].
Another topic of debate is the incidence rate of post-procedural hepatic encephalopathy after derivative treatments. The evidence further demonstrates that angioplasty without creating a bypass around the liver spares patients from complications of hepatic encephalopathy [51, 67].
TIPSS procedure
Endovascular procedures are considered as treatment options when medical treatments and angioplasty recanalization fail to alleviate portal hypertension complications and improve liver function. Moreover, early TIPSS as a first-line treatment is considered in cases with refractory portal hypertension [29, 68]. However, evidence regarding its survival benefits remains controversial [30, 52].
TIPSS in patients with BCS is technically more demanding because of obstructed HVs compared to cirrhotic patients. The procedural complication rate ranges from 0% to 56% [31]. Furthermore, pulmonary hypertension and right cardiomyopathy are absolute contraindications for TIPSS. Although the incidence rate of the above diseases is lower in patients with BCS than in those with cirrhosis, a 2D echocardiogram is required to risk stratify patients for cardiac decompensation [53, 54].
The shunt dysfunction rate between bare and covered shunts remains controversial. Bare shunts were reported to be 60-80% at 1-year post procedure [54], whereas polytetrafluoroethylene (PTFE)-covered stents were reported to have significantly better dysfunction rates [54, 55]. The patency rate of PTFE-covered stents is significantly lower in patients with BCS than in those with cirrhosis [55].
Tripathi et al reported primary patency rates at 5 years of 27% for bare stents and 70% for covered stents. In addition, the reintervention rate was 100% for bare stents and only 20% for covered stents [67].
Another topic of debate concerns the adequate size of the TIPSS. The size is directly proportional to the degree of portal decompression. A post-TIPSS portosystemic gradient of 12 mm Hg can be achieved with a 10 mm stent with a low risk of hepatic encephalopathy. Patients with BCS can tolerate a 10 mm shunt better than cirrhotic patients because of the smaller degree and extension of cirrhosis [55-57]. In patients with model for end-stage liver disease (MELD) scores > 14 and > 10, the PSPG should remain > 5 mm Hg to avoid post-TIPSS medically uncontrolled low-pressure gradient complications [58]. Post-TIPSS has been reported to be 25% for covered stents [45, 57, 68]. Liver failure usually occurs after TIPSS, with a two- to three-fold increase in LFTs and serum bilirubin. However, within 2 weeks it usually resolves thanks to the hepatic artery buffer response [59].
Assessment of coagulation and anticoagulation status
Crucial questions that need to be answered when assessing coagulation status are: First, can we rely on conventional tests to evaluate the coagulation status? Second, is the reliable indicator of INR for monitoring the effect of anticoagulants? Third, is there an evidence-based answer to the dilemma when to start anticoagulants in the presence of esophageal varices? Fourth, between vitamin K antagonist (VKA) anticoagulants and direct-acting anticoagulants (DACs) which are the preferred anticoagulants for long-term anticoagulation?
A total of 80% of BCS cases are associated with prothrombotic conditions. Using a stepwise approach, treatment anticoagulation therapy is the first-line treatment. However, only 10-20% of patients with BCS treated medically do not require any additional therapy. The time span for awaiting response to medical therapy usually extends from 2 weeks to 2 months [7, 60]. In cases of recent thrombosis and urgent need for anticoagulation, management of high-risk varices with beta-blockers may permit the application of the therapy. INR is not an accurate indicator of coagulation status in patients with BCS with thrombophilic conditions and associated liver dysfunction-related coagulopathy. In such cases, thromboelastography (TEG) is recommended to assess the dynamics, strength, and stability of thrombus formation. Of note, Jain et al reported that one-fifth of the patients were diagnosed with hypocoagulant when checked with TEG [32]. The genotype of patients and gene mutations can also determine the status of anticoagulation and the risk of overwhelming bleeding. The liver enzymes CYP2C9 and vitamin K epoxide reductase complex 1 (VKORC1) determine the action of warfarin, and mutations in these genes are associated with an increased risk of hemorrhage during warfarin treatment [33].
The impact on the metabolism of DACs in cases of hepatic failure and lack of robust evidence do not support their extensive use in BCS [32, 33].
Criteria and assessment of response to therapy
Response to treatment was defined as a restoration of blood flow across the previous obstruction and consequently improvement of LFTs, hepatomegaly, reduction of total bilirubin < 1.5 mg/dL, normalization of aspartate transaminase (AST)/alanine transaminase (ALT), no evidence of ascites without diuretic administration, no signs of portal hypertension, and hepatic encephalopathy; if the patient did not achieve all the parameters except one or two, the response was defined as partial. In the majority of patients, clinical improvement can be observed within 2 - 4 weeks. However, 30% of patients may demonstrate a slow response that may be delayed up to 5 months (Table 6) [41, 61, 62].
 Click to view | Table 6. Category of Response to Therapy or Clinical Success After Endovascular Treatment for BCS |
To date, all prognostic indices are tools to estimate survival; they are useful for clinical studies but cannot contribute and help clinicians to design individualized treatments for patients with BCS.
Recently, liver stiffness measurement (LSM), a tool that can estimate liver fibrosis, congestion, and hepatic venous pressure gradient (HVPG) in patients with liver diseases, has been used to monitor and assess the response to treatment in patients with BCS. It can be used at the presentation of patients to stratify its severity and predict the need for invasive treatment, and any reduction in LSM values after angioplasty indicates improvement of liver congestion [63, 64]. Furthermore, a case series of seven patients with BCS treated with TIPSS reported that spleen stiffness values combined with LSM values may reflect the severity of the disease at presentation and consequently may predict the need for invasive treatment [64]. Therefore, high values of both LSM and spleen stiffness measurement (SSM) can be used as reliable indicators for invasive treatment.
Implications for Future Research
Future research needs to elucidate the thrombophilic conditions and the role of their molecular pathways in the pathogenesis of BCS in the prognostic indices and criteria used to assess the severity of patients at diagnosis and consequently to risk stratification and further indicate those that need invasive therapies. Further information on the role of genotype and gene mutations in determining the coagulation status of patients with BCS may help individualize their treatment. Moreover, future studies are needed to assess the long-term extrahepatic outcomes of invasive treatments in each treatment group. Furthermore, a new common classification is urgently needed to facilitate better communication between the authors.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Author Contributions
PG contributed to conceptualization, formal analysis, investigation, methodology, software, validation, writing-original draft, and editing; GM and JA contributed to formal analysis, investigation, validation, and editing; DA contributed to conceptualization, formal analysis, investigation, methodology, software, validation, writing-original draft, editing, approval, and supervision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACLF: acute-on-chronic liver failure; AIIMS-HVOTO: All India Institute of Medical Sciences Hepatic Venous Outflow Tract Obstruction; ALF: acute liver failure; BCS: Budd-Chiari syndrome; CDSR: Cochrane Library and Database for Systematic Reviews; DACs: direct-acting anticoagulants; HCC: hepatocellular carcinoma; HV: hepatic vein; HVOTO: hepatic venous outflow tract obstruction; HVPG: hepatic venous pressure gradient; INR: international normalized ratio; IVC: inferior vena cava; LFTs: liver function tests; LSM: liver stiffness measurement; LMWH: low molecular weight heparin; LT: liver transplantation; MELD: model for end-stage liver disease; MOVC: membranous obstruction of the inferior vena cava; MPNs: myeloproliferative neoplasms; MR: magnetic resonance; NICE: National Institute for Health and Clinical Excellence; PSPG: portosystemic pressure gradient; PTFE: polytetrafluoroethylene; RCT: randomized controlled trial; SSM: spleen stiffness measurement; TIPSS: transjugular intrahepatic portosystemic shunt; US: ultrasonography; TEG: thromboelastography; VKAs: vitamin K antagonists
| References | ▴Top |
- Goel RM, Johnston EL, Patel KV, Wong T. Budd-Chiari syndrome: investigation, treatment and outcomes. Postgrad Med J. 2015;91(1082):692-697.
doi pubmed - Grus T, Lambert L, Grusova G, Banerjee R, Burgetova A. Budd-Chiari syndrome. Prague Med Rep. 2017;118(2-3):69-80.
doi pubmed - Bansal V, Gupta P, Sinha S, Dhaka N, Kalra N, Vijayvergiya R, Dutta U, et al. Budd-Chiari syndrome: imaging review. Br J Radiol. 2018;91(1092):20180441.
doi pubmed - McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20(1):3-7.
doi pubmed - Terasaki M, Kitai T, Morimoto T, Kumada K, Sasaki H, Nakano M, Sugano M, et al. Hemodynamics and hepatic energy metabolism in canine model of acute hepatic venous occlusion with mesocaval shunt. Eur Surg Res. 1994;26(1):19-27.
doi pubmed - Li Y, De Stefano V, Li H, Zheng K, Bai Z, Guo X, Qi X. Epidemiology of Budd-Chiari syndrome: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43(4):468-474.
doi pubmed - Darwish Murad S, Plessier A, Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, Trebicka J, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151(3):167-175.
doi pubmed - De Stefano V, Qi X, Betti S, Rossi E. Splanchnic vein thrombosis and myeloproliferative neoplasms: molecular-driven diagnosis and long-term treatment. Thromb Haemost. 2016;115(2):240-249.
doi pubmed - Liu L, Qi XS, Zhao Y, Chen H, Meng XC, Han GH. Budd-Chiari syndrome: current perspectives and controversies. Eur Rev Med Pharmacol Sci. 2016;20(15):3273-3281.
- Dentali F, Squizzato A, Brivio L, Appio L, Campiotti L, Crowther M, Grandi AM, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood. 2009;113(22):5617-5623.
doi pubmed - Shukla A, Shreshtha A, Mukund A, Bihari C, Eapen CE, Han G, Deshmukh H, et al. Budd-Chiari syndrome: consensus guidance of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2021;15(3):531-567.
doi pubmed - Kew MC, Hodkinson HJ. Membranous obstruction of the inferior vena cava and its causal relation to hepatocellular carcinoma. Liver Int. 2006;26(1):1-7.
doi pubmed - Okuda K. Membranous obstruction of the inferior vena cava (obliterative hepatocavopathy, Okuda). J Gastroenterol Hepatol. 2001;16(11):1179-1183.
doi pubmed - Shrestha SM, Kage M, Lee BB. Hepatic vena cava syndrome: New concept of pathogenesis. Hepatol Res. 2017;47(7):603-615.
doi pubmed - Shrestha SM. Liver cirrhosis and hepatocellular carcinoma in hepatic vena cava disease, a liver disease caused by obstruction of inferior vena cava. Hepatol Int. 2009;3(2):392-402.
doi pubmed - Rautou PE, Plessier A, Bernuau J, Denninger MH, Moucari R, Valla D. Pregnancy: a risk factor for Budd-Chiari syndrome? Gut. 2009;58(4):606-608.
doi pubmed - Cheng D, Xu H, Lu ZJ, Hua R, Qiu H, Du H, Xu X, et al. Clinical features and etiology of Budd-Chiari syndrome in Chinese patients: a single-center study. J Gastroenterol Hepatol. 2013;28(6):1061-1067.
doi pubmed - Shin N, Kim YH, Xu H, Shi HB, Zhang QQ, Colon Pons JP, Kim D, et al. Redefining Budd-Chiari syndrome: A systematic review. World J Hepatol. 2016;8(16):691-702.
doi pubmed - Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Haagsma EB, Kuipers EJ, Janssen HL. Pathogenesis and treatment of Budd-Chiari syndrome combined with portal vein thrombosis. Am J Gastroenterol. 2006;101(1):83-90.
doi pubmed - Valla DC. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018;12(Suppl 1):168-180.
doi pubmed - Ludwig J, Hashimoto E, McGill DB, van Heerden JA. Classification of hepatic venous outflow obstruction: ambiguous terminology of the Budd-Chiari syndrome. Mayo Clin Proc. 1990;65(1):51-55.
doi - Senzolo M, Cholongitas EC, Patch D, Burroughs AK. Update on the classification, assessment of prognosis and therapy of Budd-Chiari syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2(4):182-190.
doi pubmed - Van Wettere M, Bruno O, Rautou PE, Vilgrain V, Ronot M. Diagnosis of Budd-Chiari syndrome. Abdom Radiol (NY). 2018;43(8):1896-1907.
doi pubmed - Das CJ, Soneja M, Tayal S, Chahal A, Srivastava S, Kumar A, Baruah U. Role of radiological imaging and interventions in management of Budd-Chiari syndrome. Clin Radiol. 2018;73(7):610-624.
doi pubmed - Mukund A, Gamanagatti S. Imaging and interventions in Budd-Chiari syndrome. World J Radiol. 2011;3(7):169-177.
doi pubmed - He FL, Wang L, Zhao HW, Fan ZH, Zhao MF, Dai S, Yue ZD, et al. Transjugular intrahepatic portosystemic shunt for severe jaundice in patients with acute Budd-Chiari syndrome. World J Gastroenterol. 2015;21(8):2413-2418.
doi pubmed - Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8(4):453-471.
doi pubmed - Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50(1):195-203.
doi pubmed - MacNicholas R, Olliff S, Elias E, Tripathi D. An update on the diagnosis and management of Budd-Chiari syndrome. Expert Rev Gastroenterol Hepatol. 2012;6(6):731-744.
doi pubmed - Khan F, Armstrong MJ, Mehrzad H, Chen F, Neil D, Brown R, Cain O, et al. Review article: a multidisciplinary approach to the diagnosis and management of Budd-Chiari syndrome. Aliment Pharmacol Ther. 2019;49(7):840-863.
doi pubmed - Boyer TD, Haskal ZJ, American Association for the Study of Liver D. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41(2):386-400.
doi pubmed - Jain A, Dhore P, Meshram M, Bhatia S, Shukla A. Patients with Budd-Chiari syndrome have variable coagulation status on thromboelastography at diagnosis. J Clin Exp Hepatol. 2019;9(4):460-467.
doi pubmed - Shukla A, Jain A, Kahalekar V, Bendkhale S, Gogtay N, Thatte U, Bhatia S. Mutations in CYP2C9 and/or VKORC1 haplotype are associated with higher bleeding complications in patients with Budd-Chiari syndrome on warfarin. Hepatol Int. 2019;13(2):214-221.
doi pubmed - Denninger MH, Chait Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31(3):587-591.
doi pubmed - Khuroo MS, Datta DV. Budd-Chiari syndrome following pregnancy. Report of 16 cases, with roentgenologic, hemodynamic and histologic studies of the hepatic outflow tract. Am J Med. 1980;68(1):113-121.
doi - Mohanty D, Shetty S, Ghosh K, Pawar A, Abraham P. Hereditary thrombophilia as a cause of Budd-Chiari syndrome: a study from Western India. Hepatology. 2001;34(4 Pt 1):666-670.
doi pubmed - Shukla A, Sadalage A, Gupta D, Gupte A, Mahapatra A, Mazumder D, Shah C, et al. Pregnancy outcomes in women with Budd Chiari Syndrome before onset of symptoms and after treatment. Liver Int. 2018;38(4):754-759.
doi pubmed - Parekh J, Matei VM, Canas-Coto A, Friedman D, Lee WM, Acute Liver Failure Study G. Budd-chiari syndrome causing acute liver failure: A multicenter case series. Liver Transpl. 2017;23(2):135-142.
doi pubmed - Hadengue A, Poliquin M, Vilgrain V, Belghiti J, Degott C, Erlinger S, Benhamou JP. The changing scene of hepatic vein thrombosis: recognition of asymptomatic cases. Gastroenterology. 1994;106(4):1042-1047.
doi - Shukla A, Bhatt P, Gupta DK, Modi T, Patel J, Gupte A, Meshram M, et al. Budd-Chiari syndrome has different presentations and disease severity during adolescence. Hepatol Int. 2018;12(6):560-566.
doi pubmed - Zeitoun G, Escolano S, Hadengue A, Azar N, El Younsi M, Mallet A, Boudet MJ, et al. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology. 1999;30(1):84-89.
doi pubmed - Langlet P, Escolano S, Valla D, Coste-Zeitoun D, Denie C, Mallet A, Levy VG, et al. Clinicopathological forms and prognostic index in Budd-Chiari syndrome. J Hepatol. 2003;39(4):496-501.
doi - Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39(2):500-508.
doi pubmed - Garcia-Pagan JC, Heydtmann M, Raffa S, Plessier A, Murad S, Fabris F, Vizzini G, et al. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135(3):808-815.
doi pubmed - Shalimar, Kumar A, Kedia S, Sharma H, Gamanagatti SR, Gulati GS, Nayak B, et al. Hepatic venous outflow tract obstruction: treatment outcomes and development of a new prognostic score. Aliment Pharmacol Ther. 2016;43(11):1154-1167.
doi pubmed - Rautou PE, Moucari R, Escolano S, Cazals-Hatem D, Denie C, Chagneau-Derrode C, Charpignon C, et al. Prognostic indices for Budd-Chiari syndrome: valid for clinical studies but insufficient for individual management. Am J Gastroenterol. 2009;104(5):1140-1146.
doi pubmed - Mancuso A, Fung K, Mela M, Tibballs J, Watkinson A, Burroughs AK, Patch D. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol. 2003;38(6):751-754.
doi - Lupescu IG, Dobromir C, Popa GA, Gheorghe L, Georgescu SA. Spiral computed tomography and magnetic resonance angiography evaluation in Budd-Chiari syndrome. J Gastrointestin Liver Dis. 2008;17(2):223-226.
- Shukla A, Jain A. Association of acute-on-chronic liver failure with vascular liver diseases. Hepatol Int. 2019;13(4):399-402.
doi pubmed - Marudanayagam R, Shanmugam V, Gunson B, Mirza DF, Mayer D, Buckels J, Bramhall SR. Aetiology and outcome of acute liver failure. HPB (Oxford). 2009;11(5):429-434.
doi pubmed - Rathod K, Deshmukh H, Shukla A, Popat B, Pandey A, Gupte A, Gupta DK, et al. Endovascular treatment of Budd-Chiari syndrome: Single center experience. J Gastroenterol Hepatol. 2017;32(1):237-243.
doi pubmed - Rosenqvist K, Sheikhi R, Eriksson LG, Rajani R, Rorsman F, Sangfelt P, Nyman R. Endovascular treatment of symptomatic Budd-Chiari syndrome - in favour of early transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2016;28(6):656-660.
doi pubmed - Shukla A, Bhatt P, Gupta DK, Modi T, Patel J, Phadke M, Rathod K, et al. Cirrhotic cardiomyopathy is less prevalent in patients with Budd-Chiari syndrome than cirrhosis of liver. Indian J Gastroenterol. 2017;36(6):474-480.
doi pubmed - Hernandez-Guerra M, Turnes J, Rubinstein P, Olliff S, Elias E, Bosch J, Garcia-Pagan JC. PTFE-covered stents improve TIPS patency in Budd-Chiari syndrome. Hepatology. 2004;40(5):1197-1202.
doi pubmed - Gandini R, Konda D, Simonetti G. Transjugular intrahepatic portosystemic shunt patency and clinical outcome in patients with Budd-Chiari syndrome: covered versus uncovered stents. Radiology. 2006;241(1):298-305.
doi pubmed - Tripathi D, Macnicholas R, Kothari C, Sunderraj L, Al-Hilou H, Rangarajan B, Chen F, et al. Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd-Chiari syndrome. Aliment Pharmacol Ther. 2014;39(8):864-872.
doi pubmed - Sonavane AD, Amarapurkar DN, Rathod KR, Punamiya SJ. Long Term Survival of Patients Undergoing TIPS in Budd-Chiari Syndrome. J Clin Exp Hepatol. 2019;9(1):56-61.
doi pubmed - Chung HH, Razavi MK, Sze DY, Frisoli JK, Kee ST, Dake MD, Hellinger JC, et al. Portosystemic pressure gradient during transjugular intrahepatic portosystemic shunt with Viatorr stent graft: what is the critical low threshold to avoid medically uncontrolled low pressure gradient related complications? J Gastroenterol Hepatol. 2008;23(1):95-101.
doi pubmed - Casadaban LC, Parvinian A, Couture PM, Minocha J, Knuttinen MG, Bui JT, Gaba RC. Characterization of liver function parameter alterations after transjugular intrahepatic portosystemic shunt creation and association with early mortality. AJR Am J Roentgenol. 2014;203(6):1363-1370.
doi pubmed - Plessier A, Sibert A, Consigny Y, Hakime A, Zappa M, Denninger MH, Condat B, et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology. 2006;44(5):1308-1316.
doi pubmed - Shukla A, Bhatia SJ. Outcome of patients with primary hepatic venous obstruction treated with anticoagulants alone. Indian J Gastroenterol. 2010;29(1):8-11.
doi pubmed - Li WD, Yu HY, Qian AM, Rong JJ, Zhang YQ, Li XQ. Risk factors for and causes and treatment of recurrence of inferior vena cava type of Budd-Chiari syndrome after stenting in China: A retrospective analysis of a large cohort. Eur Radiol. 2017;27(3):1227-1237.
doi pubmed - Mukund A, Pargewar SS, Desai SN, Rajesh S, Sarin SK. Changes in liver congestion in patients with Budd-Chiari syndrome following endovascular interventions: assessment with transient elastography. J Vasc Interv Radiol. 2017;28(5):683-687.
doi pubmed - Dajti E, Ravaioli F, Colecchia A, Marasco G, Vestito A, Festi D. Liver and spleen stiffness measurements for assessment of portal hypertension severity in patients with Budd Chiari syndrome. Can J Gastroenterol Hepatol. 2019;2019:1673197.
doi pubmed - Raby N, Karani J, Meire H, Michell M, Howard E. Budd-Chiari syndrome: shunt selection and post-operative assessment. Clin Radiol. 1989;40(6):586-590.
doi - Wang Q, Li K, He C, Yuan X, Luo B, Qi X, Guo W, et al. Angioplasty with versus without routine stent placement for Budd-Chiari syndrome: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(9):686-697.
doi - Tripathi D, Sunderraj L, Vemala V, Mehrzad H, Zia Z, Mangat K, West R, et al. Long-term outcomes following percutaneous hepatic vein recanalization for Budd-Chiari syndrome. Liver Int. 2017;37(1):111-120.
doi pubmed - Qi X, Guo W, He C, Zhang W, Wu F, Yin Z, Bai M, et al. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome: techniques, indications and results on 51 Chinese patients from a single centre. Liver Int. 2014;34(8):1164-1175.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


