| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 7, July 2022, pages 273-281
High Expression of Heat Shock Protein Family D Member 1 Predicts Poor Prognosis of Esophageal Cancer
Jing Lva, Xian Wei Wangb, Xiao Kang Suna, Jun Rong Yanga, Pei Rui Chena, c
aDepartment of Cardiothoracic Surgery, People’s Hospital of Deyang City, Deyang City, Sichuan Province 618001, China
bDepartment of Pathology, People’s Hospital of Deyang City, Deyang City, Sichuan Province 618001, China
cCorresponding Author: Pei Rui Chen, Department of Cardiothoracic Surgery, People’s Hospital of Deyang City, Deyang City, Sichuan Province 618001, China
Manuscript submitted January 6, 2021, accepted January 26, 2021, published online July 29, 2022
Short title: HSPD1 Predicts Poor Prognosis of EC
doi: https://doi.org/10.14740/jocmr4424
| Abstract | ▴Top |
Background: Heat shock protein family D (Hsp60) member 1 (HSPD1) has been reported as a potential survival-related biomarker in some cancers. However, the correlation between HSPD1 expression with prognosis and clinical features of esophageal cancer (EC) is poorly understood. Our research aimed to explore the clinical and prognostic significance of HSPD1 expression in EC patients.
Methods: In our study, HSPD1 expression was detected by immunochemistry in 87 EC tissue specimens and 20 normal cancerous peripheral tissue specimens. Meanwhile, we also analyzed the expression of HSPD1 in EC by The Cancer Genome Atlas (TCGA) database. Then Chi-squared and Fisher’s exact tests and Wilcoxon signed-rank test and logistic regression models were separately used to test the correlation between clinical characteristics and HSPD1 expression in our and TCGA cohort. Moreover, we evaluated the value of HSPD1 in prognosis by Kaplan-Meier curves and Cox analysis. Finally, gene set enrichment analysis (GSEA) was performed using the data accessed from TCGA.
Results: The results showed that HSPD1 was overexpressed in EC, and the expression was related to histological type, histological grade, N classification, and clinical stage. Moreover, Kaplan-Meier curves and Cox analysis indicated that high expression of HSPD1 correlated with poor prognosis, and HSPD1 was an independent risk factor for EC. GSEA identified pathways involved in cysteine and methionine metabolism, spliceosome, selenoamino acid metabolism, mismatch repair, RNA degration, DNA replication, and cell cycle as differentially enriched in ECs with high HSPD1 expression.
Conclusions: Our results suggest that HSPD1 is expressed at high levels in EC, and has potential to be used as a novel biomarker for the prognosis of patients with EC.
Keywords: HSPD1; Esophageal cancer; Prognosis
| Introduction | ▴Top |
Esophageal cancer (EC) is the ninth most common cancer and sixth most common cause of cancer-related death globally [1]. Every year more than 400,000 people suffer from EC and the incidence rates are increasing rapidly [2], with about 40% of that in China alone [3]. Although the prognosis and survival have improved, the 5-year survival rate remains low [4]. Therefore, it is essential to find more novel potential prognostic biomarkers for improving the prognoses of EC patients.
Heat shock proteins (HSPs) are groups of genetically highly conserved proteins involved in maintaining cell homeostasis during normal physiology [5]. According to the different molecular weights, these proteins have been classified, including HSPB1 (HSP27), DNAJB1 (HSP40), HSPD1 (HSP60), HSPA4 (HSP70), HSP90AA1 (HSP90) and HSPH (HSP110) [6]. Besides their cytoprotective effects, previous studies have demonstrated HSPs involved in the development, progression, metastasis and drug resistance of cancers [7]. Potential clinical roles of some HSPs in ECs have been reported. For example, Hsp27 activation increased ALDH activity, chemoresistance and tumor initiation in esophageal squamous cell carcinoma (ESCC) cell lines, and is considered as a prognostic indicator in ESCC [8, 9]. High expression of HSP47 is associated with poor prognosis in patients with ESCC [10]. HSP90a has potential clinical application as a predictor of response to chemoradiotherapy, and might be an independent prognostic factor for ESCC [11, 12]. However, the clinical significance of HSPD1 in EC is still not clear. Therefore, our research aimed to explore the clinicopathological significance of HSPD1 expression in EC patients by a combined method of immunohistochemistry and bioinformatics.
| Materials and Methods | ▴Top |
Tumor specimens and clinical data collection
We retrospectively searched our institutional database for EC patients between January 2013 and December 2017. Patients who underwent chemotherapy and/or radiotherapy prior to surgery or biopsy were excluded from the study. Then, a total of 87 paraffin-embedded EC tissue specimens and 20 adjacent non-neoplastic tissue specimens were collected. The pathological diagnosis of each tissue specimen was confirmed by at least two pathologists. Medical records of each patient were used to extract data including age, gender, histological type, histological grade, stage classification, T classification, N classification and M classification. The date of diagnosis was set as the starting point and the date of death or last date of follow-up as the end point. The present study was approved by the Ethics Committee of People’s Hospital of Deyang City (Deyang, China) and all human tissue samples were obtained following written informed consent.
Immunohistochemistry
Immunohistochemical staining with HSPD1 (1:100 dilution; Abcam) was accomplished using Dako Link 48 autostainer (DAKO) following the manufacturer’s instructions. Immunostaining was evaluated independently by two senior pathologists who blinded to the clinical data, on at least 10 random fields at 400 magnification and cytoplasmic stained cells were counted. The immunohistochemical expression was scored as follows: high expression, > 50% and low expression, < 50% of the cancer cells positive staining.
Data collection from The Cancer Genome Atlas (TCGA) database
The data of EC patients and mRNA expression profiles (169 cases, including 10 normal samples) was downloaded from the TCGA database (https://cancergenome.nih.gov/). The expression difference of HSPD1 was shown as a box plot. P values below 0.05 were considered statistically significant. The associations between clinical features and HSPD1 expression were assessed by the Wilcoxon signed-rank test and logistic regression.
Gene set enrichment analysis (GSEA)
In this study, GSEA was performed to find the significant pathways between low expression and high expression group of HSPD1 by using the GSEA software (4.1.0). The high and low expression groups were defined as the median value of expression level of HSPD1. The normalized enrichment score (NES) was acquired by investigating permutations for 1,000 times. A gene set is considered to signify the statistical significance when a normal P-value is < 0.05 as well as false discovery rate (FDR) is < 0.05. The graphical results are shown in one diagram by using R software (V.4.0.2).
Statistical analysis
The relationships between HSPD1 expression and clinicopathological features were analyzed using the Chi-square test or Fisher’s exact test in our study. Kaplan-Meier curves were performed, and the differences in the overall survival were compared by log-rank test. Univariate Cox analysis was conducted to select the significant related parameters. Then, the multivariate Cox analysis was applied to assess the independent prognostic factor for overall survival of EC patients. The result of multivariate Cox analysis was shown as a forest plot by using the survminer R package. All statistical analyses were performed using R software (V.4.0.2).
| Results | ▴Top |
High HSPD1 expression in EC
In total, 169 EC and adjacent non-neoplastic tissue samples from the TCGA database were included in the current comparison. As shown in Figure 1, the HSPD1 expression was elevated in EC tissues compared with adjacent non-neoplastic tissues (P = 0.0237). In our study, we performed immunohistochemical analysis to assess HSPD1 expression in EC tissues and normal adjacent non-neoplastic tissues (Fig. 2). HSPD1 was mainly expressed in the cytoplasm. In the EC tissues, high expression of HSPD1 was observed in 54% (47/87) tumor samples. While in the adjacent non-neoplastic tissues, 10% (2/20) of samples exhibited high HSPD1 expression. The statistical result suggested that the expression of HSPD1 in EC tissues was higher (P < 0.001, Table 1), which was consistent with the results of TCGA database.
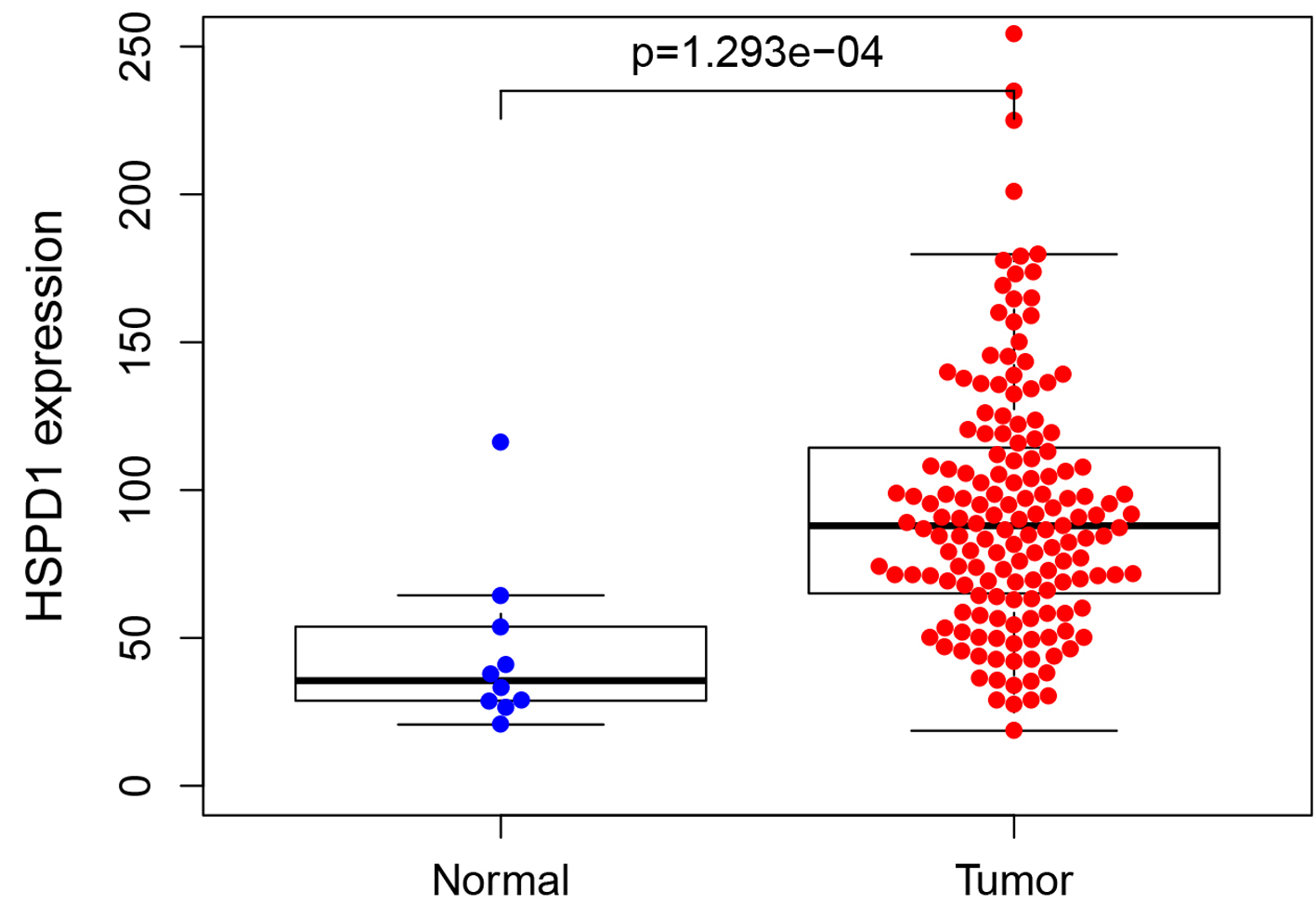 Click for large image | Figure 1. Expression of HSPD1 in esophagus cancer patients based on TCGA data. The expression level of HSPD1 in esophagus cancer tissues was significantly higher than that in adjacent non-neoplastic tissues (P = 0.0237). HSPD1: heat shock protein family D (Hsp60) member 1; TCGA: The Cancer Genome Atlas. |
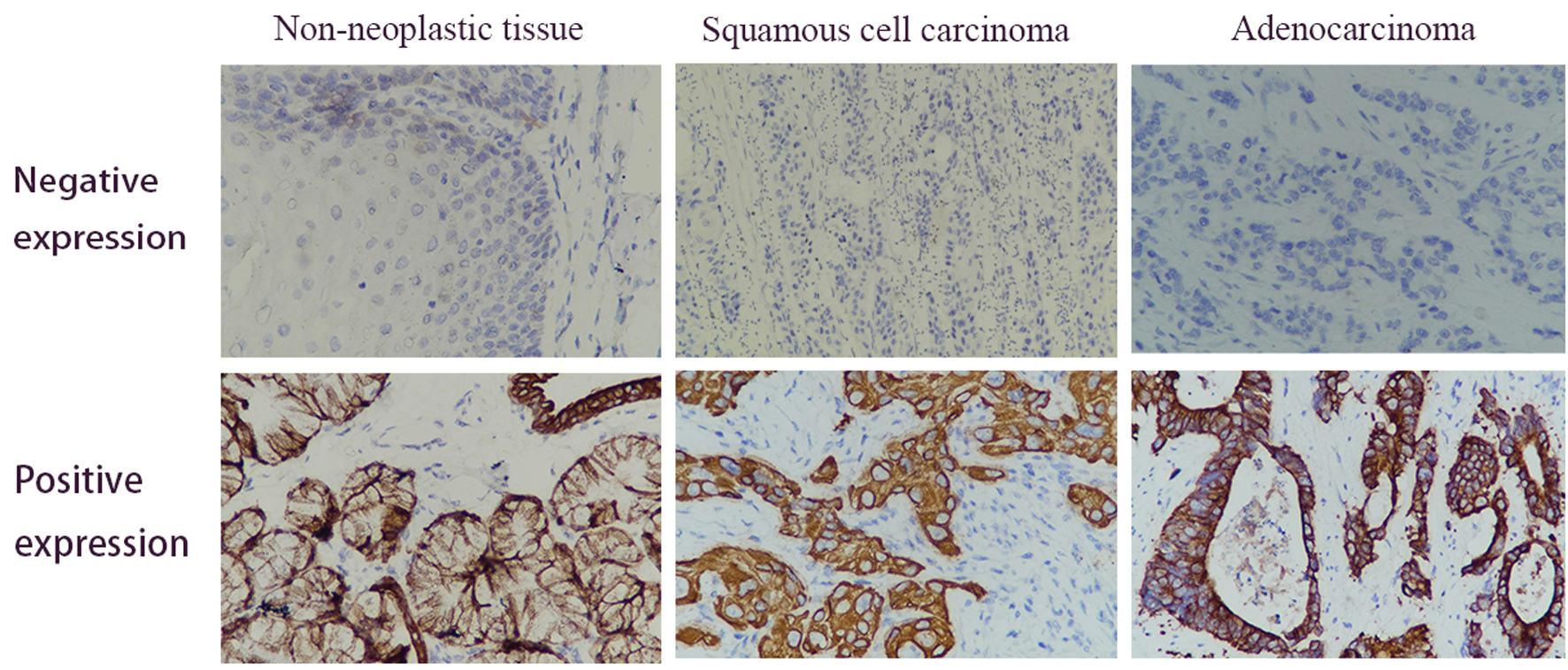 Click for large image | Figure 2. Immunohistochemical analysis of the HSPD1 expression in esophagus cancer and adjacent non-neoplastic tissues. Positive expression of HSPD1 was mainly found in the cytoplasm (magnification, × 400). HSPD1: heat shock protein family D (Hsp60) member 1. |
 Click to view | Table 1. HSPD1 Expression in Esophagus Cancer Tissues and Adjacent Non-Neoplastic Tissues |
Associations between HSPD1 expression and clinical features in EC patients
In TCGA cohort, the connections between the clinicopathological characteristics and the expression of HSPD1 were analyzed and summarized in Figure 3 and Table 2. HSPD1 expression was notably related with histological type (P = 0.001), clinical stage (P = 0.024), T classification (P = 0.003), N classification (P = 0.007) and M classification (P = 0.008) (Fig. 3). Univariate analysis using logistic regression showed that high expression of HSPD1 in EC was significantly associated with high N classification (odds ratio (OR) = 2.109 for N0 vs. N1-3), adenocarcinoma pathological type (OR = 0.488 for adenocarcinoma vs. squamous carcinoma). In our study, the expression of HSPD1 was highly associated with the clinical stage (I-II vs. III-IV, P = 0.001, Table 3), N classification (N0 vs. N1-3, P = 0.014, Table 3) and histological grade (well vs. moderately/poorly, P < 0.001, Table 3).
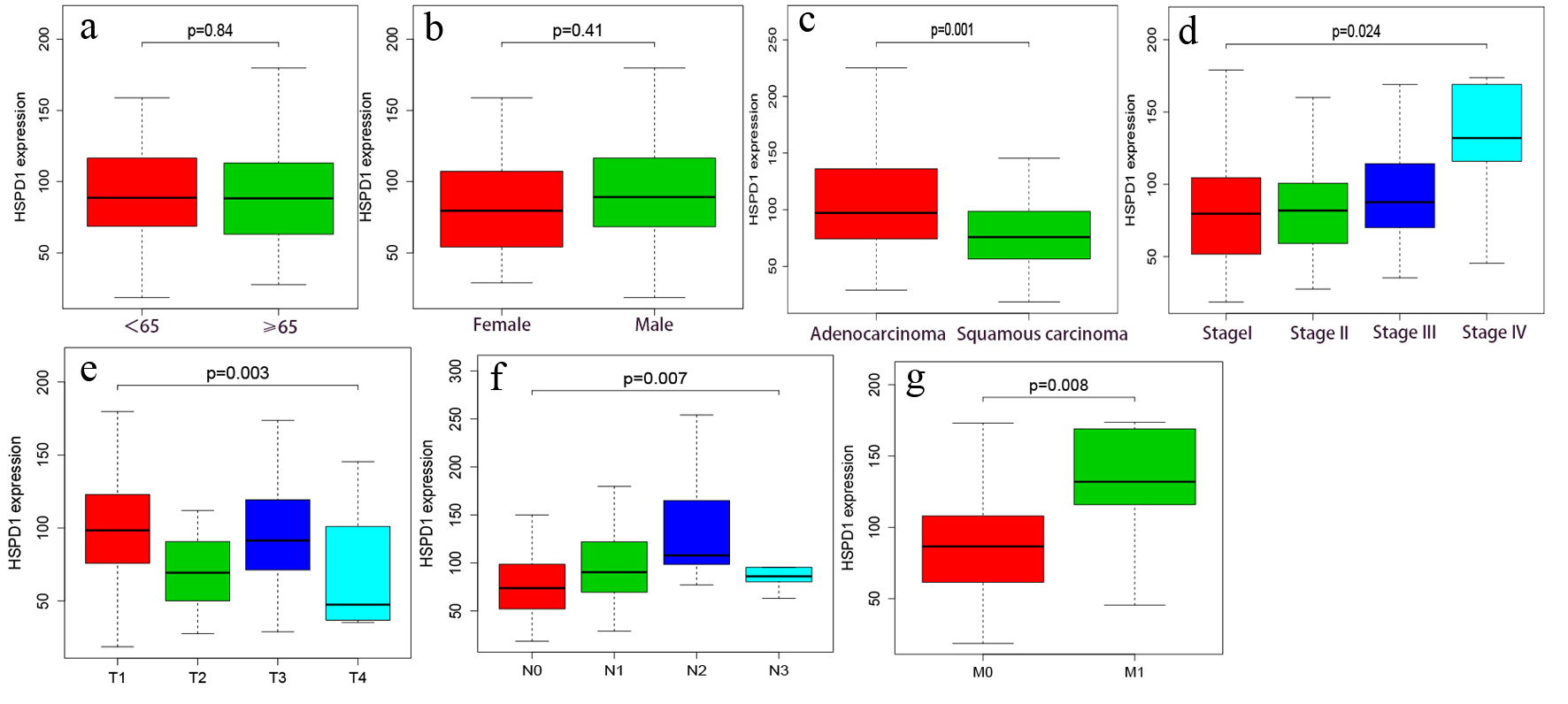 Click for large image | Figure 3. Association of HSPD1 expression with clinical variables based on TCGA data. (a) Age. (b) Gender. (c) Histological type. (d) Clinical stage. (e) T classification. (f) N classification. (g) M classification. HSPD1: heat shock protein family D (Hsp60) member 1; TCGA: The Cancer Genome Atlas. |
 Click to view | Table 2. Logistic Regression of HSPD1 Expression and Clinicopathological Characteristics in TCGA Database |
 Click to view | Table 3. Associations Between HSPD1 Expression and Clinicopathological Characteristics in Our Esophagus Cancer Patients |
Expression of HSPD1 is associated with the overall survival
Firstly, Kaplan-Meier survival analysis along with the log-rank test was performed based on expression level of HSPD1 in EC cohort from TCGA database, and we found that EC patients with high HSPD1 expression were closely associated with poor overall survival than those with a low HSPD1 expression (P = 0.012; Fig. 4a). This relationship was validated in the EC cohort from our study (P = 0.008, Fig. 4b). Secondly, high HSPD1 expression, clinical stage, N classification and M classification were selected by univariate Cox proportional hazards regression analysis. Furthermore, the result of multivariate Cox proportional hazards regression analysis suggested that high HSPD1 expression (hazard ratio (HR) = 1.572) and advanced clinical stage (HR = 2.281) were independent prognostic factors for EC patients in TCGA cohort (Fig. 5a). These findings were consistent with the results based on our EC cohort (HR = 1.595 for high HSPD1 expression and HR = 1.679 for advanced clinical stage, Fig. 5b).
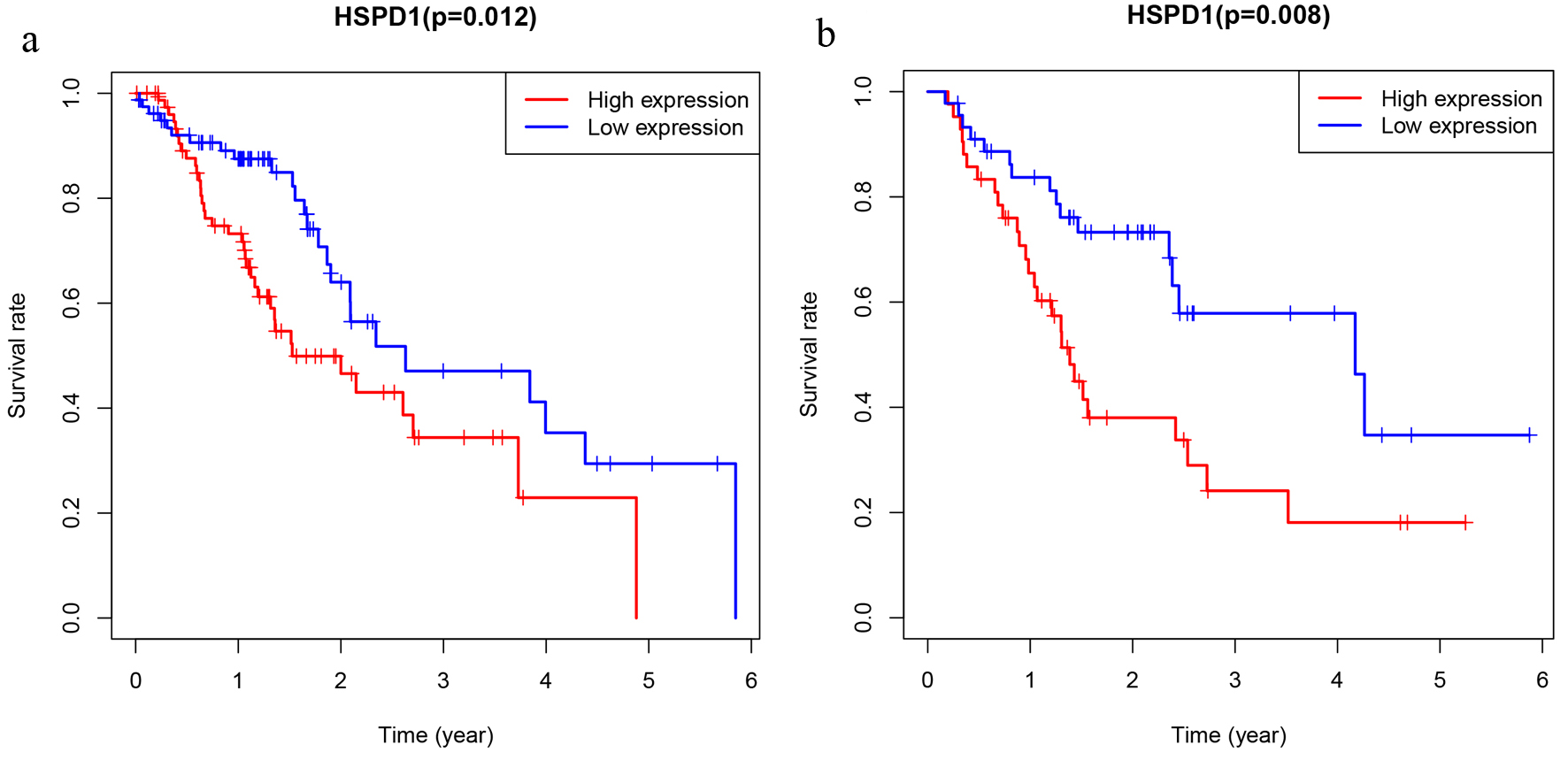 Click for large image | Figure 4. The prognostic significance of HSPD1 in esophagus cancer. Kaplan-Meier method and log-rank test were performed based on expression level of HSPD1 in esophagus cancer cohort from TCGA database (a) and our study (b). HSPD1: heat shock protein family D (Hsp60) member 1; TCGA: The Cancer Genome Atlas. |
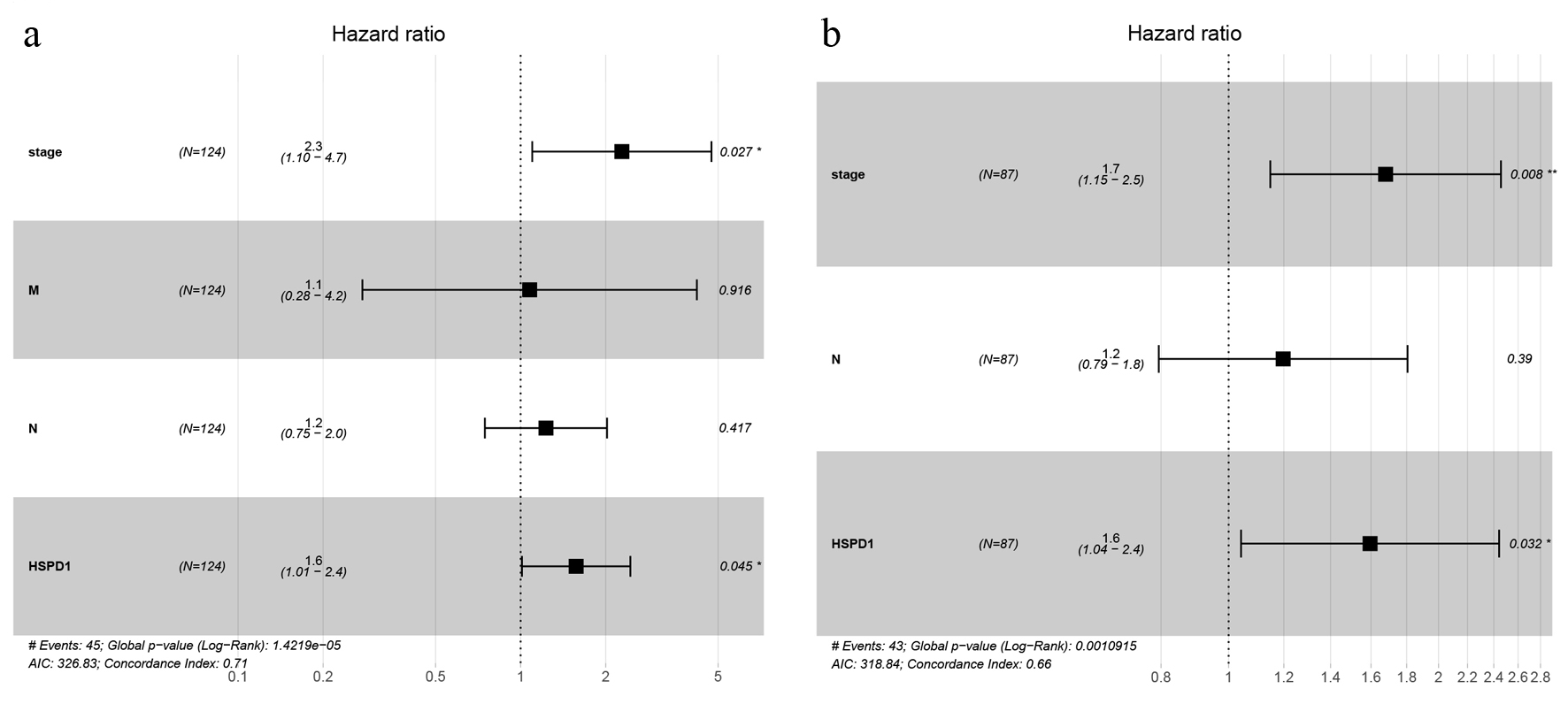 Click for large image | Figure 5. Forest plot of multivariate Cox regression analyses of overall survival in esophagus cancer cohort from TCGA database (a) and our study (b). TCGA: The Cancer Genome Atlas. |
GSEA identifies HSPD1-associated signaling pathways
In order to explore potential biological pathways that were activated in different groups, GSEA was performed. GSEA revealed significant differences in the enrichment of the MSigDB collection (c2. cp. kegg. v7.1.symbols. gmt). As shown in Figure 6, gene sets related to cysteine and methionine metabolism, spliceosome, selenoamino acid metabolism, RNA degradation, cell cycle, mismatch repair and DNA replication were associated with the HSPD1 high expression phenotype.
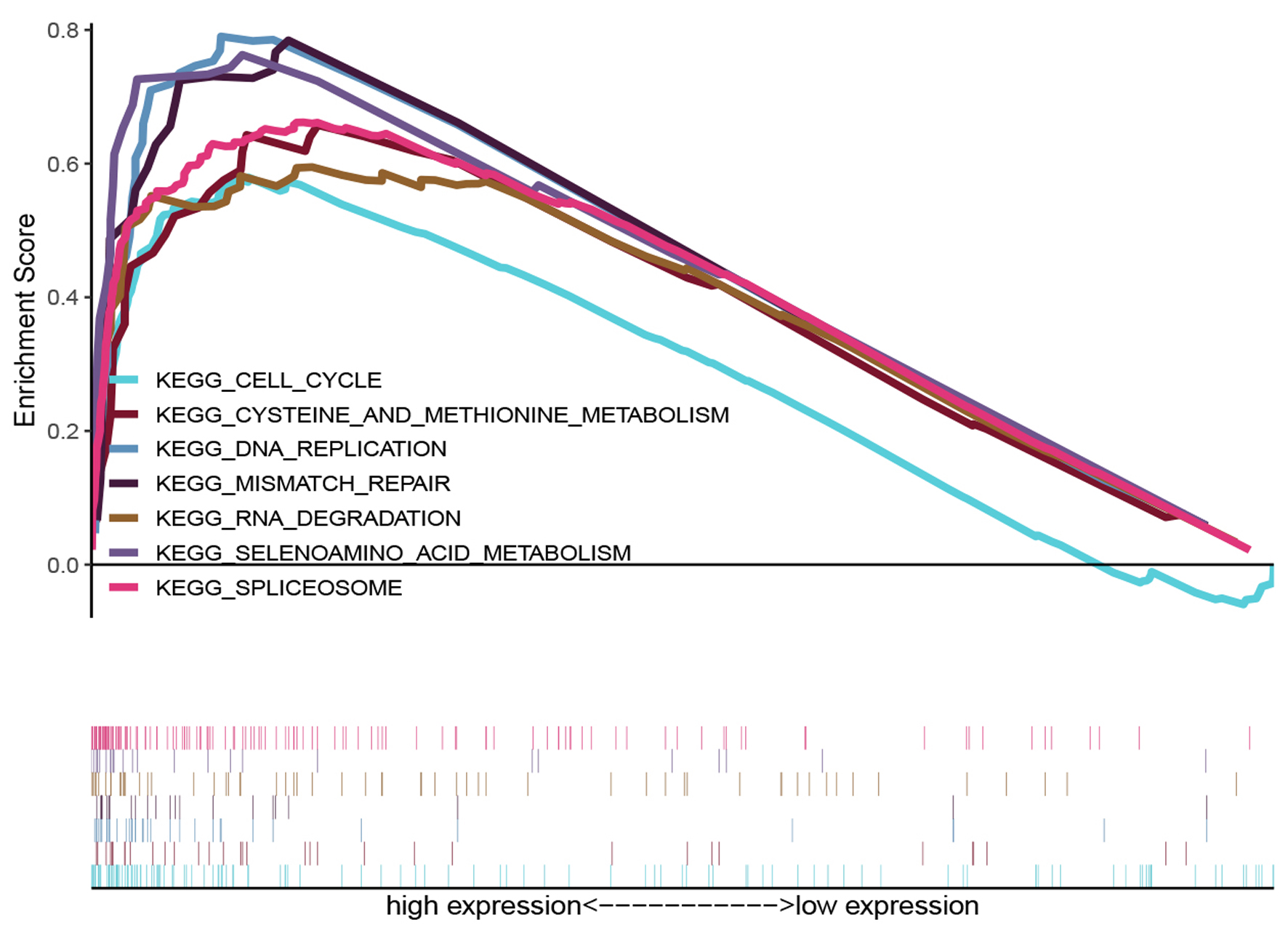 Click for large image | Figure 6. KEGG enrichment plots from GSEA. The GSEA results revealed that genes involved in cysteine and methionine metabolism, spliceosome, selenoamino acid metabolism, mismatch repair, RNA degration, DNA replication and cell cycle were differentially enriched in HSPD1-associated esophagus cancer. HSPD1: heat shock protein family D (Hsp60) member 1; GSEA: gene set enrichment analysis. |
| Discussion | ▴Top |
HSPD1 is a nuclear-encoded mitochondrial protein, primarily but not exclusively localized in the mitochondrial matrix [13]. Together with co-chaperonin HSP10, it facilitates correct folding and assembly of imported proteins in the mitochondria, and as a signaling molecule that activates a class of immunoreaction [14]. Besides, it has pro-inflammatory functions, and plays a role of dual pro-survival [15, 16] and pro-apoptosis [17, 18] functions. Recently, HSPD1 has been considered as a prognostic biomarker for poor overall survival involved in several types of cancers [19-21]. However, in EC, the relationship between HSPD1 expression and tumor histopathology or clinical prognosis is controversial. In an early study, Faried et al [22] reported that positive HSPD1 expression in ESCC contributed to the induction of apoptosis and correlated with favorable prognosis. In contrast, in a study from China, expression of HSPD1 was significantly increased in ESCC, and associated with poor disease survival [23]. Therefore, the correlation between HSPD1 and EC remains to be further explored.
HSPD1 was expressed at markedly higher levels in several kinds of cancers, such as oral [20], prostate [24], gastric [19], colorectal [25] or cervical [26] cancer. However, low HSPD1 expression was observed in ovarian cancer [27]. In the present study, we found that EC tissues exhibited high HSPD1 expression in comparison with adjacent non-neoplastic tissue, which was consistent with the expression status of HSPD1 in TCGA database. Together with our study in EC, these results suggested that HSPD1 expression levels may be associated with tumorigenesis in most types of human cancer. Further, we found that HSPD1 expression was markedly associated with advanced clinical stage, more lymph node metastasis and worse histological grade. The result of univariate analysis using logistic regression models suggested high expression of HSPD1 was significantly associated with high N classification and adenocarcinoma pathology type in TCGA cohort, which was partly consistent with our findings. We further evaluated the association between HSPD1 expression and overall survival of EC patients in TCGA database, and found HSPD1 expression was negatively correlated with overall survival time in EC patients. Furthermore, similar result has shown in the overall survival curve of our cohort. Univariate and multivariate Cox proportional hazards regression analyses of both TCGA database and our cohort indicated HSPD1 expression was an independent factor for poor prognosis in EC patients. Generally, high HSPD1 expression is a credible biomarker for predicting poor prognosis in EC patients.
Recently, HSPD1 has been found in many extramitochondrial sites, including the extracellular surface, cell surface, intracellular vesicles, nucleus, extracellular fluid, and even the cytoplasm [28]. It has been reported that HSPD1, especially which consists in cytosolic, is involved in an increased ability of metastasis and cell survival of various cancers [19, 21, 29]. Tsai et al [30] reported that cytosolic HSPD1 interacts with β-catenin to promote metastasis through enhancing transcriptional activity and increasing protein levels of β-catenin in head and neck cancer. Moreover, it has been reported that HSPD1 represses E-cadherin expression at transcriptional and translational levels through RelA activation and may contributes to metastasis in buccal mucosa squamous cell carcinoma (BMSCC) cells [20]. Downregulation of HSPD1 could induce the apoptosis of gastric cancer cells and was negatively correlated with the MEK/ERK signaling in vitro [31]. Also, inhibition of HSPD1 could suppress the proliferation of glioblastoma cells through the ROS/AMPK/mTOR pathway [32]. Although many potential functions have been presented for HSPD1 in various types of cancer, no report relates HSPD1 to biological role in EC so far. To further evaluate the roles of HSPD1 in EC, we performed GSEA using TCGA data. GSEA showed that genes involved in cysteine and methionine metabolism, spliceosome, selenoamino acid metabolism, RNA degradation, cell cycle, mismatch repair and DNA replication were associated with the HSPD1 high expression phenotype. It has been reported that methionine levels might be vital for promoting proliferation and drug resistance of cancers [33, 34]. Moreover, methionine deprivation can stop tumors from growing in various cancers, and chemo-sensitize cancer cells [35-38]. A recent study showed that H2S-producing enzyme cystathionine γ-lyase (CTH) which was involved in the metabolism of cysteine and methionine could generate H2S to promote prostate cancer metastasis and progression by IL-1β/NF-κB signaling pathways [39]. To date, several studies have reported that alternative splicing provides the potential to generate diversity at RNA and protein levels from an apparently limited number of loci in the genome. Dysregulation of alternative splicing characterizes many cancers and is sufficient to drive disease initiation, progression, and therapeutic response [40-42]. Moreover, mismatch repair capacity, RNA degration, DNA replication and cell cycle pathway are also the critical mechanism in cancer progression [43-46]. Although we have demonstrated that the value of HSPD1 expression is a potential reliable molecular marker for the prognosis in EC, some limitations of our study should be noted. First, the sample used in this research is limited. Second, further exploration needs to be carried out in the future to verify the detailed molecular mechanisms of HSPD1 expression in EC.
Conclusions
HSPD1 expression is up-regulated in EC tissues, and positively associated with clinical progression in EC patients. In patients with EC, the expression levels of HSPD1 and clinical stage were independent prognostic factors. Further studies should be performed to evaluate the diagnostic and prognostic role of HSPD1 and its potential molecular role as a therapeutic target.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by scientific research project of Sichuan Health Commission (20PJ248).
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Not applicable.
Author Contributions
PC, WX, XS, JY, and JL performed the experiments; WX performed the statistical analysis; and PC designed the study, analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Global Burden of Disease Cancer, Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505-527.
doi pubmed - Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400-412.
doi - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383-2396.
doi - Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631-677.
doi pubmed - Sannam Khan R, Khurshid Z, Akhbar S, Faraz Moin S. Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: an update. Proteomes. 2016;4(4):41.
doi pubmed - Calderwood SK, Gong J. Heat shock proteins promote cancer: it's a protection racket. Trends Biochem Sci. 2016;41(4):311-323.
doi pubmed - Liu CC, Chou KT, Hsu JW, Lin JH, Hsu TW, Yen DH, Hung SC, et al. High metabolic rate and stem cell characteristics of esophageal cancer stem-like cells depend on the Hsp27-AKT-HK2 pathway. Int J Cancer. 2019;145(8):2144-2156.
doi pubmed - Xie YH, Li LY, He JZ, Xu XE, Liao LD, Zhang Q, Xie JJ, et al. Heat shock protein family B member 1 facilitates ezrin activation to control cell migration in esophageal squamous cell carcinoma. Int J Biochem Cell Biol. 2019;112:79-87.
doi pubmed - Lee HW, Kwon J, Kang MC, Noh MK, Koh JS, Kim JH, Park JH. Overexpression of HSP47 in esophageal squamous cell carcinoma: clinical implications and functional analysis. Dis Esophagus. 2016;29(7):848-855.
doi pubmed - Yan H, Li B, Fan T, Jiang S, Wang R, Sun M. Clinical significance of serum dynamics of HSP90a level in esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy. Cancer Biomark. 2017;19(2):185-192.
doi pubmed - Wang S, Du Z, Luo J, Wang X, Li H, Liu Y, Zhang Y, et al. Inhibition of heat shock protein 90 suppresses squamous carcinogenic progression in a mouse model of esophageal cancer. J Cancer Res Clin Oncol. 2015;141(8):1405-1416.
doi pubmed - Soltys BJ, Gupta RS. Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol. 2000;194:133-196.
doi - Quintana FJ, Cohen IR. The HSP60 immune system network. Trends Immunol. 2011;32(2):89-95.
doi pubmed - Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105(24):2899-2904.
doi pubmed - Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103(13):1787-1792.
doi pubmed - Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18(8):2040-2048.
doi pubmed - Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, et al. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18(8):2049-2056.
doi pubmed - Li XS, Xu Q, Fu XY, Luo WS. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS One. 2014;9(9):e107507.
doi pubmed - Kang BH, Shu CW, Chao JK, Lee CH, Fu TY, Liou HH, Ger LP, et al. HSPD1 repressed E-cadherin expression to promote cell invasion and migration for poor prognosis in oral squamous cell carcinoma. Sci Rep. 2019;9(1):8932.
doi pubmed - Zhou J, Li XL, Chen ZR, Chng WJ. Tumor-derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget. 2017;8(59):100781-100790.
doi pubmed - Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40(18):2804-2811.
doi pubmed - Chen JH, Chen LM, Xu LY, Wu MY, Shen ZY. [Expression and significance of heat shock proteins in esophageal squamous cell carcinoma]. Zhonghua Zhong Liu Za Zhi. 2006;28(10):758-761.
- Cappello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003;23(2B):1325-1331.
- Cappello F, Bellafiore M, Palma A, David S, Marciano V, Bartolotta T, Sciume C, et al. 60KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem. 2003;47(2):105-110.
doi pubmed - Castle PE, Ashfaq R, Ansari F, Muller CY. Immunohistochemical evaluation of heat shock proteins in normal and preinvasive lesions of the cervix. Cancer Lett. 2005;229(2):245-252.
doi pubmed - Schneider J, Jimenez E, Marenbach K, Romero H, Marx D, Meden H. Immunohistochemical detection of HSP60-expression in human ovarian cancer. Correlation with survival in a series of 247 patients. Anticancer Res. 1999;19(3A):2141-2146.
- Fan G, Tu Y, Wu N, Xiao H. The expression profiles and prognostic values of HSPs family members in Head and neck cancer. Cancer Cell Int. 2020;20:220.
doi pubmed - Cappello F, David S, Rappa F, Bucchieri F, Marasa L, Bartolotta TE, Farina F, et al. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. 2005;5:139.
doi pubmed - Tsai YP, Yang MH, Huang CH, Chang SY, Chen PM, Liu CJ, Teng SC, et al. Interaction between HSP60 and beta-catenin promotes metastasis. Carcinogenesis. 2009;30(6):1049-1057.
doi pubmed - Tong WW, Tong GH, Kong H, Liu Y. The tumor promoting roles of HSP60 and HIF2alpha in gastric cancer cells. Tumour Biol. 2016;37(7):9849-9854.
doi pubmed - Tang H, Li J, Liu X, Wang G, Luo M, Deng H. Down-regulation of HSP60 suppresses the proliferation of glioblastoma cells via the ROS/AMPK/mTOR pathway. Sci Rep. 2016;6:28388.
doi pubmed - Modis K, Coletta C, Asimakopoulou A, Szczesny B, Chao C, Papapetropoulos A, Hellmich MR, et al. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-beta-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146-156.
doi pubmed - Ham MS, Lee JK, Kim KC. S-adenosyl methionine specifically protects the anticancer effect of 5-FU via DNMTs expression in human A549 lung cancer cells. Mol Clin Oncol. 2013;1(2):373-378.
doi pubmed - Sinha R, Cooper TK, Rogers CJ, Sinha I, Turbitt WJ, Calcagnotto A, Perrone CE, et al. Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in TRAMP mice. Prostate. 2014;74(16):1663-1673.
doi pubmed - Strekalova E, Malin D, Good DM, Cryns VL. Methionine deprivation induces a targetable vulnerability in triple-negative breast cancer cells by enhancing TRAIL Receptor-2 Expression. Clin Cancer Res. 2015;21(12):2780-2791.
doi pubmed - Mecham JO, Rowitch D, Wallace CD, Stern PH, Hoffman RM. The metabolic defect of methionine dependence occurs frequently in human tumor cell lines. Biochem Biophys Res Commun. 1983;117(2):429-434.
doi - Liu H, Zhang W, Wang K, Wang X, Yin F, Li C, Wang C, et al. Methionine and cystine double deprivation stress suppresses glioma proliferation via inducing ROS/autophagy. Toxicol Lett. 2015;232(2):349-355.
doi pubmed - Wang YH, Huang JT, Chen WL, Wang RH, Kao MC, Pan YR, Chan SH, et al. Dysregulation of cystathionine gamma-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20(10):e45986.
doi - Yang X, Coulombe-Huntington J, Kang S, Sheynkman GM, Hao T, Richardson A, Sun S, et al. Widespread expansion of protein interaction capabilities by alternative splicing. Cell. 2016;164(4):805-817.
doi pubmed - Dvinge H, Bradley RK. Widespread intron retention diversifies most cancer transcriptomes. Genome Med. 2015;7(1):45.
doi pubmed - Climente-Gonzalez H, Porta-Pardo E, Godzik A, Eyras E. The functional impact of alternative splicing in cancer. Cell Rep. 2017;20(9):2215-2226.
doi pubmed - Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93-115.
doi pubmed - Xu XC. Risk factors and gene expression in esophageal cancer. Methods Mol Biol. 2009;471:335-360.
doi pubmed - Uchida N, Kumimoto H, Nishizawa K, Tokumasu S, Harada H, Shimada Y, Ishizaki K. Mismatch repair and microsatellite instability in esophageal cancer cells. Int J Cancer. 2001;91(5):687-691.
doi - Chang NW, Huang YP. The RNA degradation pathway is involved in PPARalpha-modulated anti-oral tumorigenesis. Biomedicine (Taipei). 2019;9(4):27.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


