| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 5, May 2019, pages 326-331
Prevalence of Documented Excessive Weight Gain Among Adolescent Girls and Young Women Using Depot Medroxyprogesterone Acetate
Preeyaporn Jirakittidula, b, Chotiros Somyapraserta, Surasak Angsuwathanaa
aDepartment of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
bCorresponding Author: Preeyaporn Jirakittidul, Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wanglang Road, Bangkok 10700, Thailand
Manuscript submitted February 22, 2019, accepted March 19, 2019
Short title: Weight Gain in Adolescents Using DMPA
doi: https://doi.org/10.14740/jocmr3792
| Abstract | ▴Top |
Background: Depot medroxyprogesterone acetate (DMPA) is an accessible contraception with high efficacy among adolescents. However, concern of weight gain can impede DMPA use. The objective of this study was to determine prevalence of excessive weight gain associated with DMPA injection in young women and its predicting factor.
Methods: This retrospective chart review included young women aged 10 - 24 years who had used DMPA and attended at Family Planning and Reproductive Medicine Unit, Siriraj Hospital for at least 6-month period during January 2010 to June 2016. Baseline clinical data, weight at beginning of contraception, and weight at 6 and 12 months thereafter were reviewed. Excessive weight was defined as weight gain of > 5% of their baseline weight at 6 months. Various baseline variables were compared between groups with and without excessive weight gain.
Results: Among 231 DMPA users in this study, there were 28 women (12.1%, 95% confidence interval (CI): 7.8 - 16.3) who had an excessive weight gain at 6 months. Age, baseline body mass index, or race did not affect the likelihood of excessive weight gain. The excessive weight gain group had significant higher proportion of nulliparity, unmarried status and DMPA-ever used history compared to another one. Six of 13 (46.2%) excessive weight gainers at 6 months who continued DMPA use had gain even more weight (> 10% of their baseline weigh) at 12 months.
Conclusions: The majority of adolescent girls using DMPA had no excessive weight gain in 6 months. However, DMPA users who had excessive weight at 6 months were at high risk of gaining even more weight at 1 year.
Keywords: DMPA; Contraception; Weight gain; Adolescent; Young women
| Introduction | ▴Top |
Adolescent pregnancy is a major public health problem in many developing countries, including Thailand [1-3]. Recently a national survey reported adolescent birth rate in Thai was 117 per 1,000 deliveries [2, 3]. Pregnancy in this vulnerable age group not only increases the risk of adverse pregnancy outcomes, but also negatively influences the quality of their life. Previous study reported that the vast majority of adolescent pregnancy had not intended to become pregnant. In addition, a substantial number of them are unwanted and might be terminated by undergoing an unsafe abortion. Thus, effective contraception is very important in this age group [1].
Several policy statements recommend the long-acting reversible contraception (LARC), including intrauterine devices and subdermal implants, be used as the first-line contraceptive option in adolescent girls and young women [4]. Currently, it seems that only 1-4% of them are utilizing these contraceptive methods. These may be due to that there are several barriers for teens to use LARC, such as lack of method offering, its cost and accessibility. Nowadays, the most frequently used contraceptive methods in adolescents are combined oral contraception (COC) and depot medroxyprogesterone acetate (DMPA) injection [5].
DMPA is an accessible contraception with high efficacy and privacy. However, concerns of weight gain associated with DMPA use can impede contraceptive initiation and can lead to early discontinuation among the users. Weight gain has been reported as the most important reason for contraceptive discontinuation by 40% of adolescents receiving DMPA [6]. Proper counseling prior initiation of the method might improve the perception of weight change and reduce ongoing discontinuation. Several existing evidences demonstrated a significantly increased weight in DMPA users with the prevalence of excessive weight gain up to one-fourth in all age groups [6-8]. A recent meta-analysis was still inconclusive about an excessive weight gain associated with DMPA use, especially in adolescent girls and young women who still have a physiologic growth with different basal metabolic rate [9].
An excessive weight gain is an important concerning adverse effect among DMPA users that can deter contraceptive initiation and continuation, and the evidences of weight gain in adolescent users were still limited, highlighting the need to explore this point of view. Accordingly, the objective of the present study was to determine the prevalence of excessive weight gain and its associated factors among adolescent girls and young women who used DMPA injection.
| Materials and Methods | ▴Top |
The protocol of the present retrospective study was approved by the Siriraj Institutional Review Board (COA no. si583/2017). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The requirement to obtain written informed consent was waived given the retrospective nature of this study. This retrospective chart review included all young women aged 10 - 24 years who had started using DMPA injection and attended at the Family Planning and Reproductive Medicine Unit, Siriraj Hospital during the study period from January 2010 to June 2016. The exclusion criteria were women who had delivered infant of less than 3 months, had chronic medical disorders affecting bodily metabolism, had concurrent use of other hormonal drugs, had a pregnancy within the study period, and had inadequate data about body weight either at baseline or 6 months after starting DMPA.
Collected data included clinical characteristics and demographic data, such as age, race, marital status, parity, last child delivery, and previous history of hormonal contraceptive use. Body weight of each visit was obtained from the medical record. The weight of all women was measured with a calibrated digital scale at each visit. An excessive weight gain was defined by using a percentage of weight change from baseline that was classified into two subtypes: an early excessive weight gain which was defined as an increasing weight of more than 5% from their baseline weight at 6 months after DMPA initiation, and a later excessive weight gain which was defined as an increasing weight of more than 10% from their baseline weight at 10 months after DMPA initiation [8].
The sample size was calculated based on the prevalence of an early excessive weight gain in adolescent and younger DMPA users of 28.2% [7]; a total of 216 women would be required for an alpha of 0.05 with an absolute precision error of 6%.
STATA statistical software (version 11; StataCorp LLC, College Station, TX, USA) was used to analyze data. Data were presented as mean ± standard deviation (SD), number (n) and percent, or percent and 95% confidence interval (CI) as appropriate. The data were analyzed using Chi-square test or Fisher’s exact test for categorical data, and t-test or Mann-Whitney U test for continuous data. A regression analysis was performed to compare data between groups. Statistical significance was considered when a P value was less than 0.05.
| Results | ▴Top |
Initially, a total of 1,819 women started DMPA injection during the studying period and met the inclusion criteria, but 1,588 were excluded according to the above mentioned exclusion criteria. Finally, 231 women were recruited in this study (Fig. 1). The clinical characteristics and demographic data of all patients were shown in Table 1. They were 20.17 ± 2.64 years of age and 39.8% of them were adolescent. Almost all of them were Thai. Their average body mass index (BMI) was 21.00 ± 3.49 kg/m2 and few of them were obese (2.46%). The majority of the patients were married or in union. Of all patients, only 8.23% were nulliparous and nearly 15% had ever used DMPA. The mean body weight of all DMPA users at baseline and at 6 months, 12 months after method initiation were demonstrated in Figure 2.
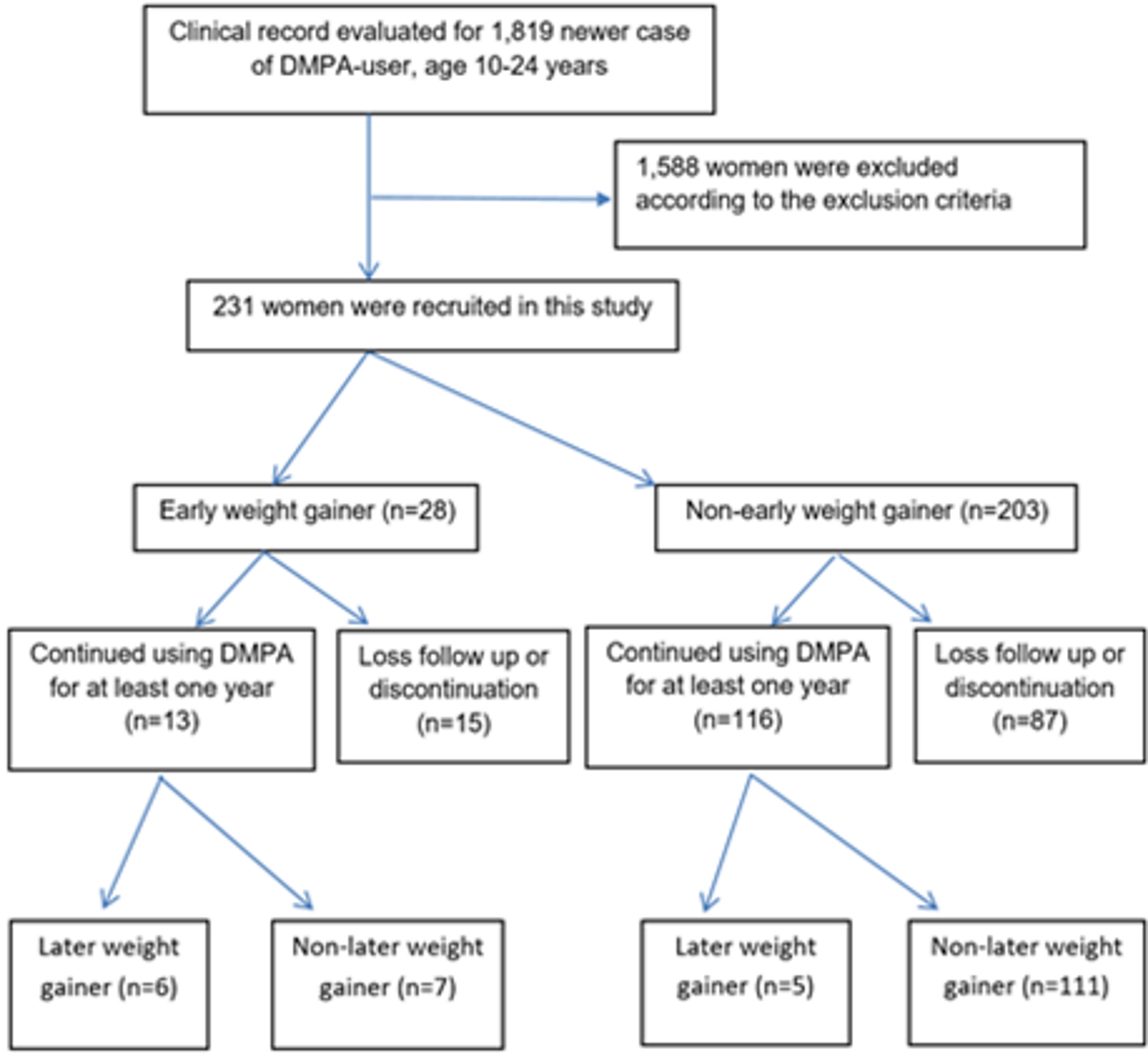 Click for large image | Figure 1. Flow of the study. DMPA: depot medroxyprogesterone acetate. |
 Click to view | Table 1. Baseline Characteristics of 231 DMPA Users |
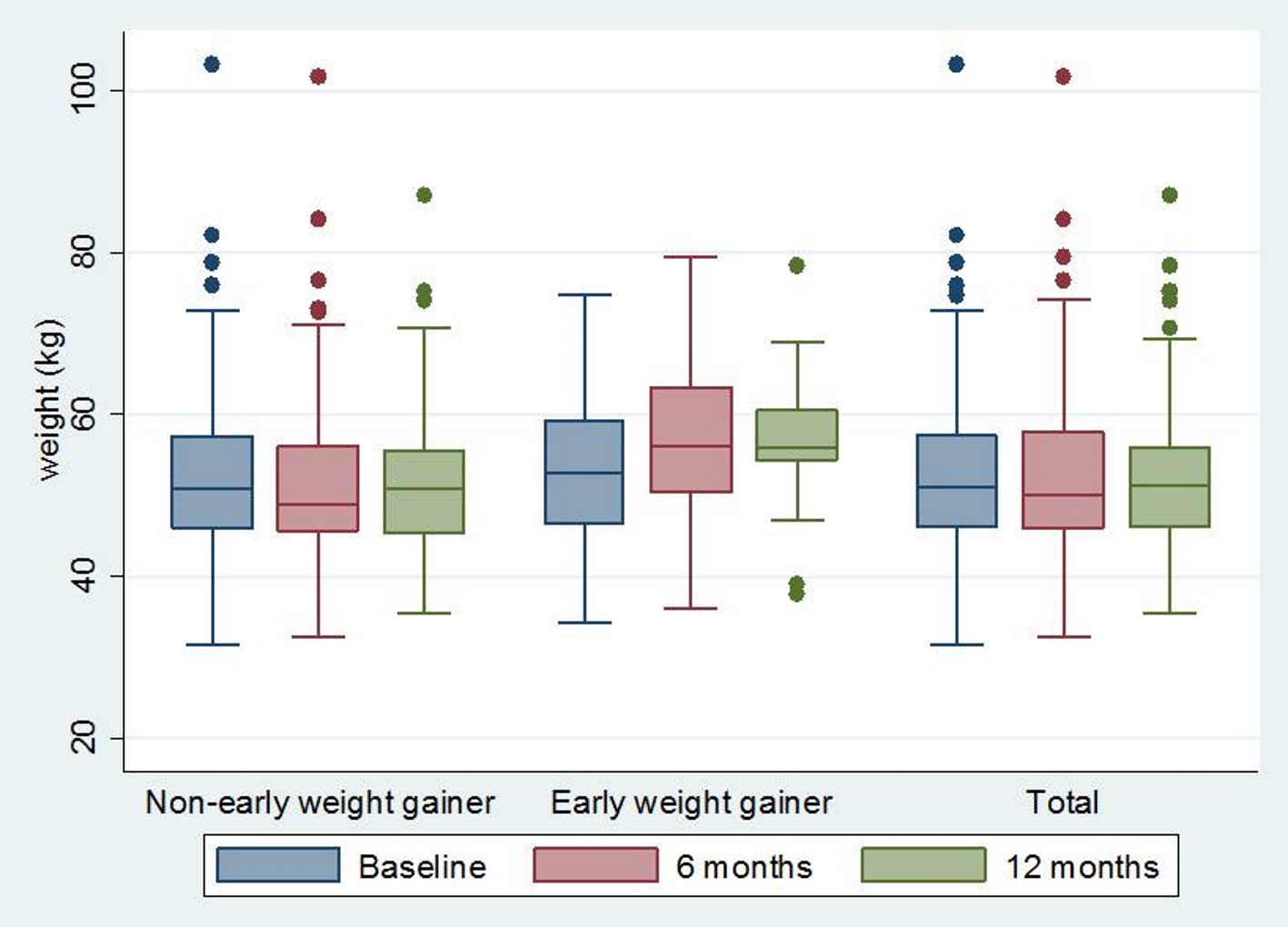 Click for large image | Figure 2. Body weight at baseline, 6 months after DMPA initiation and 12 months after DMPA initiation. DMPA: depot medroxyprogesterone acetate. |
Among 231 DMPA users in this study, there were 28 women who had more than a 5% weight gain after 6 months of DMPA use. The prevalence of an early excessive weight gain was 12.1% (95% CI: 7.8 - 16.3). Clinical characteristics and demographic data of 28 women who had more than a 5% weight gain after 6 months of DMPA use and 203 women who had less than a 5% weight gain after 6 months of DMPA use were also shown in Table 1. The excessive weight gain group had significant higher proportion of nulliparous, unmarried status and DMPA-ever used history compared to another one. The estimated risk for early excessive weight gain increased in women who were nulliparous (relative risk (RR) 3.71, 95% CI: 1.81 - 7.60), were in unmarried or non-in union status (RR 3.33, 95% CI: 1.39 - 7.94), and had ever used DMPA (RR 2.84, 95% CI: 1.40 - 5.73), as presented in Table 2.
 Click to view | Table 2. Estimated Risk of Having Early Excessive Weight Gain in 231 DMPA Users |
A total of 129 women in this study continued using DMPA for at least one year, among whom 13 women had an excessive weight gain at 6 months and 116 women had less gain. The prevalence of a later excessive weight gain was 8.5% (95% CI: 3.6 - 13.4).The trend of weight changes of each individual woman with excessive weight gaining at 12 months after DMPA initiation was shown in Figure 3. Nearly half of the early gainers had gained even more weight (> 10% of their baseline weigh) at 12 months. However, only 4% of non-early gainer had a later excessive weight gain (Fig. 1). Early gainers had 10-fold higher risk of further later excessive weight gain than non-early weight gainers (RR 10.71, 95% CI 3.79 - 30.26, P < 0.001).
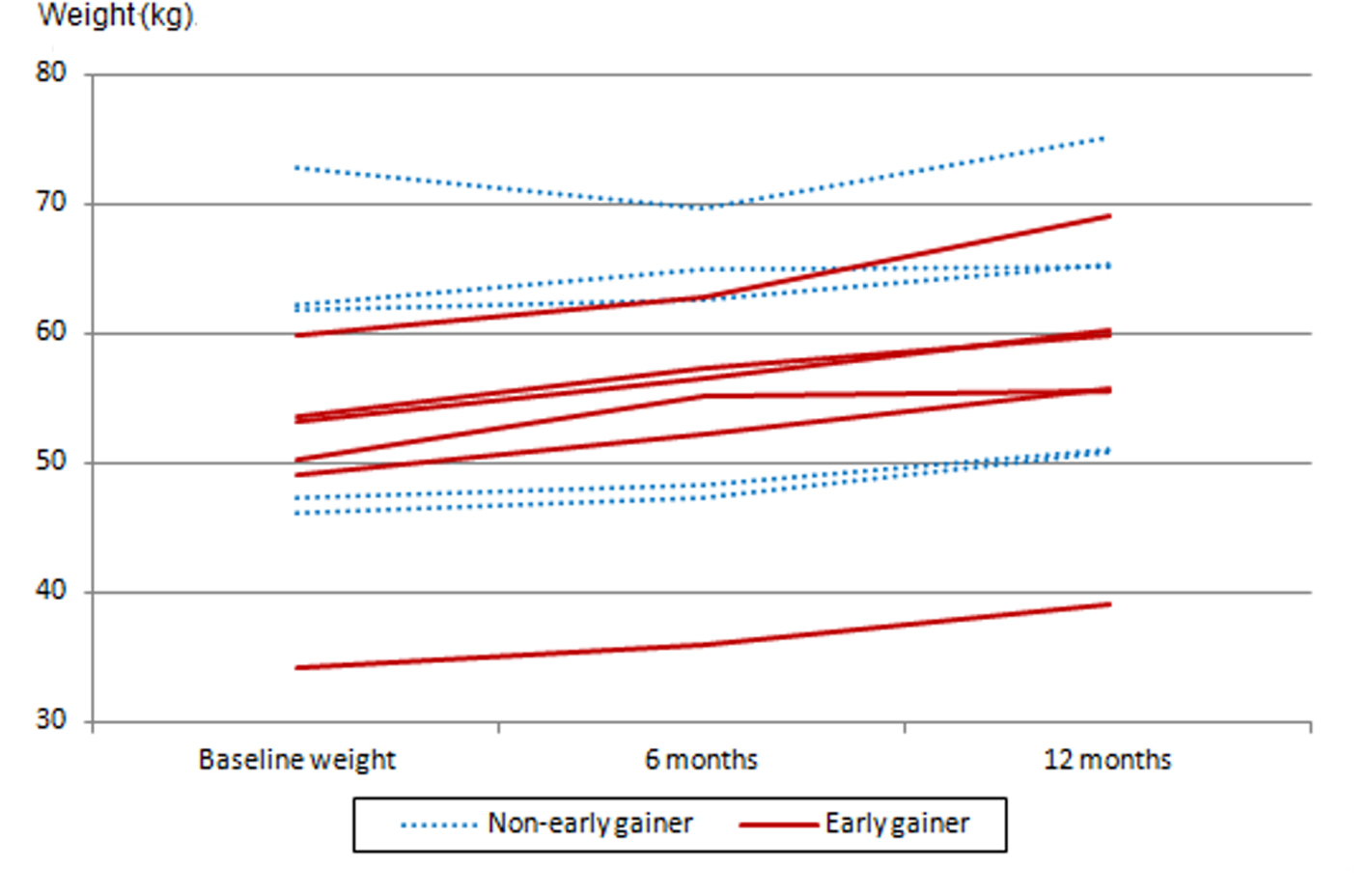 Click for large image | Figure 3. The trend of weight changes of each individual woman who has an excessive weight gain at 12 months after DMPA initiation. DMPA: depot medroxyprogesterone acetate. |
| Discussion | ▴Top |
This study focused on determining the impact of DMPA use on the occurrence of an excessive weight gain because large amount of gaining might lead to overweight and obesity which can further lead to long-term health problems. We used an increasing weight of more than 5 and 10% of their baseline weight at 6 and 12 months after DMPA initiation according to a significant impact on reproductive health when 5-10% of the weight was changed [10, 11].
The overall prevalence of an excessive weight gain among adolescent girls and younger women in this study were 12.1% at 6 months after DMPA use and 8.5 % at 12 months after DMPA use, respectively. The lower prevalence in our study might be due to the difference in age, race and baseline BMI [6-8]. In the present study, we included only women aged 10 - 24 years and most of them were Thai and had normal BMI prior to initiating DMPA injection. However, our findings were consistent with a recent systematic review, which concluded that there were a small number of women who had weight gaining associated with progestin-only contraceptive use [9].
During the study, we also identified risk factors of an early excessive weight gain as the secondary objective and our data suggested that risk factors for having an early excessive weight gain were nulliparity, unmarried status, and prior DMPA use that were different from the previous reports. However, the predicting risk factors of substantial weight gain in DMPA user were difficult to identify and still inconsistent among several studies. Some previous studies reported that the difference in an excessive weight gain depended on race and ethnicities, which was more prevalent in black than in white, Hispanic, and Asian populations [6, 12]. Some study reported increased risk of excessive weight gain on DMPA use that was associated with baseline BMI or initial overweight [7, 10]. Some studies found an increasing weight during DMPA, which was related to parity [7]. However, some studies did not find any factors that were associated with the risk of weight gain [8]. However, our data suggested that an early excessive weight was related to continued excessive weight gain and can be used to predict it, which was consistent with recent reports [7, 13].
From these findings, our study supports that only a few adolescent girls and young women on DMPA suffered from an excessive weight gain associated with this contraceptive method. However, adolescent girls and younger women who had an early excessive weight gain at 6 months after DMPA use were at 10-time increased risk for continued excessive gain at 1 year, which could have negatively impacted on further health. In addition, weight gain was not a uniform finding among all women using DMPA injection. Only non-married status, nulliparity and history of DMPA ever use were associated with an increasing risk of excessive weight gain.
A limitation of the present study was its retrospective study design that could not control for numerous confounds and usually faces the problem of missing data.
Conclusions
In summary, the majority of adolescent girls and young women using DMPA had no excessive weight gain. The potential for excessive weight gain with DMPA use is probable for some women who were nulliparous, were in unmarried or non-in union status, and had ever used DMPA. In addition, our finding further supports that an early excessive weight at 6 months was a useful clinical predictor to identify women at risk for continued weight gaining at 1 year. Thus, early gainers should be informed about this impact and be advised for routine exercise and dietary control.
Acknowledgments
The authors would like to thank all staff members of the Family Planning and Reproductive Medicine Unit, Faculty of Medicine Siriraj Hospital, Mahidol University for their contribution to taking care of the study participants.
Funding
The present study was financially supported by Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand (Grant number: R016131015).
Conflict of Interest
All authors declare no personal or professional conflict of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in the present report.
Informed Consent
Not applicable.
Author Contributions
PJ contributed to project development, data collection, data analysis, and manuscript writing. CS contributed to project development, data collection, and manuscript editing. SA contributed to project development, supervising, and manuscript editing.
| References | ▴Top |
- Lee L, Upadhya KK, Matson PA, Adger H, Trent ME. The status of adolescent medicine: building a global adolescent workforce. Int J Adolesc Med Health. 2016;28(3):233-243.
doi pubmed - Areemit R, Thinkhamrop J, Kosuwon P, Kiatchoosakun P, Sutra S, Thepsuthammarat K. Adolescent pregnancy: Thailand's national agenda. J Med Assoc Thai. 2012;95(Suppl. 7):134-142.
- Sriprasert I, Chaovisitsaree S, Sribanditmongkhol N, Sunthornlimsiri N, Kietpeerakool C. Unintended pregnancy and associated risk factors among young pregnant women. Int J Gynaecol Obstet. 2015;128(3):228-231.
doi pubmed - Diedrich JT, Klein DA, Peipert JF. Long-acting reversible contraception in adolescents: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(4):364.e1-364.e12.
doi pubmed - Borovac-Pinheiro A, Surita FG, D'Annibale A, Pacagnella RC, Pinto ESJL. Adolescent Contraception Before and After Pregnancy-Choices and Challenges for the Future. Rev Bras Ginecol Obstet. 2016;38(11):545-551.
doi pubmed - Bonny AE, Britto MT, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA). J Pediatr Adolesc Gynecol. 2004;17(2):109-115.
doi pubmed - Le YC, Rahman M, Berenson AB. Early weight gain predicting later weight gain among depot medroxyprogesterone acetate users. Obstet Gynecol. 2009;114(2 Pt 1):279-284.
doi pubmed - Risser WL, Gefter LR, Barratt MS, Risser JM. Weight change in adolescents who used hormonal contraception. J Adolesc Health. 1999;24(6):433-436.
doi - Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, Helmerhorst FM. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016;8:CD008815.
doi - Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2002;15(2):79-82.
doi - Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481-1486.
doi pubmed - Vickery Z, Madden T, Zhao Q, Secura GM, Allsworth JE, Peipert JF. Weight change at 12 months in users of three progestin-only contraceptive methods. Contraception. 2013;88(4):503-508.
doi pubmed - Bonny AE, Secic M, Cromer B. Early weight gain related to later weight gain in adolescents on depot medroxyprogesterone acetate. Obstet Gynecol. 2011;117(4):793-797.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


