| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 8, August 2016, pages 575-581
Predictors of Acute Hemodynamic Decompensation in Early Sepsis: An Observational Study
Young Im Leea, b, c, Robert L. Smitha, b, Yevgeniya Gartshteyna, Sophia Kwona, Erin J. Carahera, Anna Nolana, b, d
aDivision of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, New York University School of Medicine, New York, NY, USA
bPulmonary and Critical Care Sections of Veterans Administration, New York Harbor Healthcare System, New York, NY, USA
cMount Sinai Beth Israel, Icahn School of Medicine at Mount Sinai, New York, NY, USA
dCorresponding Author: Anna Nolan, Medicine and Environmental Medicine, NYU School of Medicine, Division of Pulmonary, Critical Care and Sleep, New Bellevue, 7N Room 24, 462 1st Avenue, New York, NY 10016, USA
Manuscript accepted for publication May 25, 2016
Short title: Hypotension in Early Sepsis
doi: http://dx.doi.org/10.14740/jocmr2597w
| Abstract | ▴Top |
Background: The study of sepsis is hindered by its heterogeneous time course and evolution. A subgroup of patients with severe sepsis develops shock soon after the initiation of treatment while others present hypotensive. We sought to determine the incidence of hypotension after the initiation of treatment for sepsis, and characterize their clinical features and course.
Methods: A retrospective review of electronic medical record of all septic patients (n = 542) that met the definition of septic shock within 24 hours of admission (2011 - 2012) at an urban Veteran Affairs Hospital was performed. Subjects either had 1) initial normotension (INT) with hypotension developing within 24 hours or 2) initial hypotension (IH). Logistic regression was used to model associated factors of INT/IH.
Results: INT occurred in 62 patients (11%) with average initial blood pressure of 120/71 mm Hg and developed hypotension to 79/48 mm Hg. IH was identified in 52 patients (10%) with average presenting blood pressure of 81/46 mm Hg. INT showed evidence of increased sympathetic tone with significantly higher heart rate, blood pressure and temperature. INT patients were younger, more frequently on alpha-blockers, and more likely septic from pneumonia compared to IH patients. INT and IH patients had similar timing of antibiotic initiation, amount of 24-hour fluid resuscitation, vasopressor use, organ dysfunction and mortality at 28 days. Using alpha-blockers, being Caucasian, and having higher temperatures were independent predictors of INT.
Conclusion: INT is a distinctive presentation of septic shock characterized by rapid deterioration during early treatment. By further studying this subgroup, mediators of septic shock may be identified that clarify pathophysiology and provide timely targeted treatment.
Keywords: Sepsis; Septic shock; Vascular hypotension; Rehydration solutions; Fluid therapy; Adrenergic effects
| Introduction | ▴Top |
Sepsis remains a challenge to the healthcare system and is associated with frequent hospitalizations and high mortality [1-3]. The pathophysiology of sepsis has been intensively studied [2, 4]. However, clinical studies of sepsis are often hindered by its heterogeneous time course [4, 5]. Sepsis treatment studies have emphasized early recognition and treatment is critical to successful outcomes [6-8]. The effectiveness of interventions, such as fluid or antibiotic administration, is time-dependent [7-10]. The patient with sepsis who presents with hypotension is intuitively a different clinical phenotype than the patient who presents over a more prolonged time course [8, 10-12]. This distinction may be important in terms of prognosis and may influence the interventions deployed. We examined these two phenotypes of septic shock presentation within the first day of hospital admission and their response to standard of care (SOC).
We performed a retrospective review of patients presenting with sepsis, severe sepsis, and septic shock. We compared patients who presented with initial hemodynamic stability before evolving into septic shock to those who presented initially with septic shock in terms of initial findings, management, and outcomes. We investigated the associated patient-specific and treatment factors involved with the evolution of severe sepsis to septic shock. Early recognition of patients with severe sepsis who are at risk for deterioration into septic shock could potentially be useful in management decisions.
| Materials and Methods | ▴Top |
Study design and population
We conducted a retrospective cohort study at the Veterans Affairs (VA) New York Harbor Healthcare System, an urban tertiary referral center. Data were from the electronic medical record (EMR) of all patients that were identified as having been admitted with the diagnosis of sepsis, severe sepsis, or septic shock in 2011 and 2012, (n = 542, Fig. 1). We excluded patients who 1) remained stable and had normotensive blood pressure during the initial 24 h of their hospitalization, 2) became hypotensive from etiologies other than sepsis or 3) had received significant sepsis treatment, specifically fluids or antibiotics, prior to hospital admission from other hospitals/nursing facilities and 4) did not have complete blood pressure data available. Study was approved by the hospital’s Institutional Review Board and granted a waiver of informed consent.
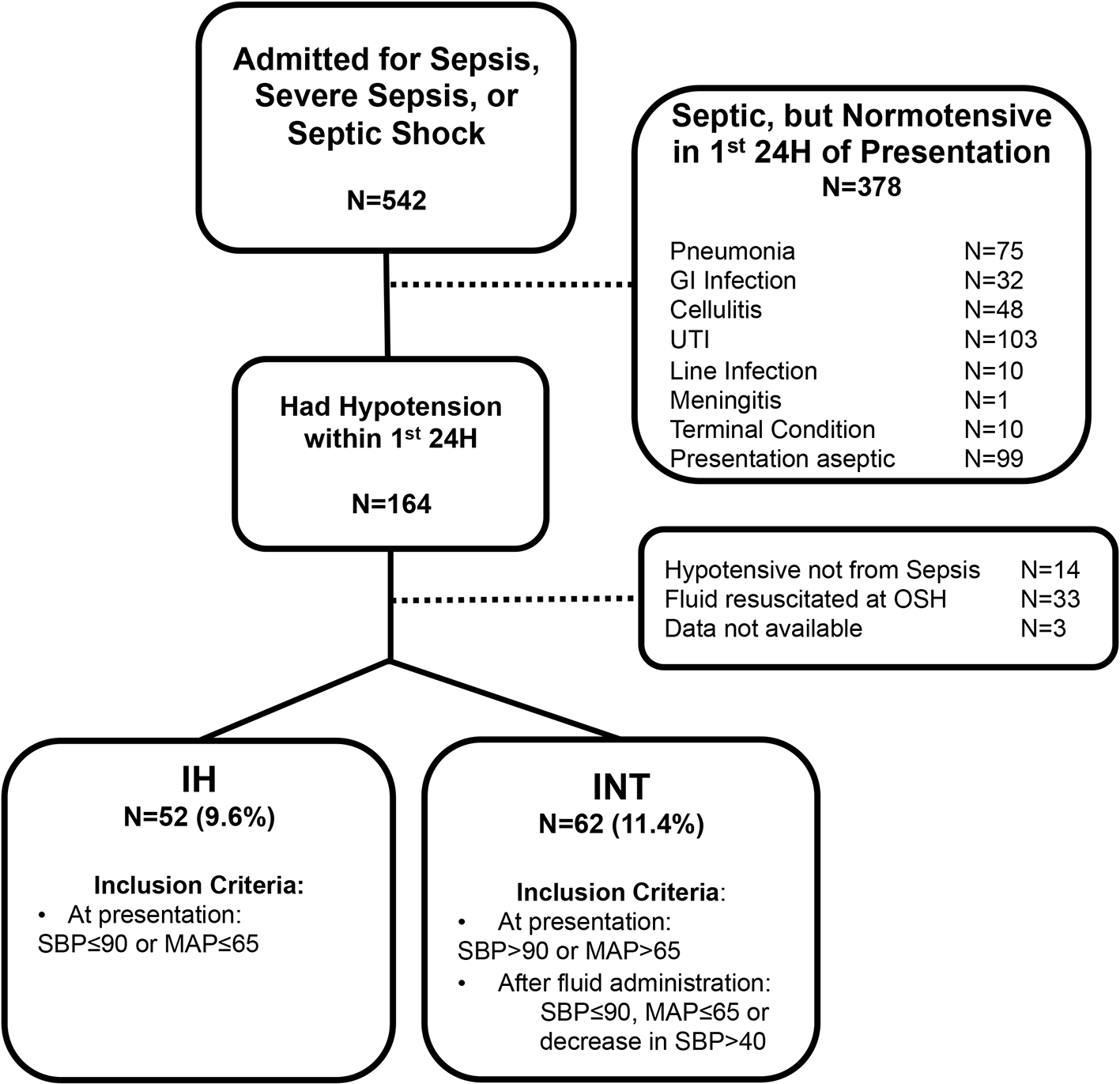 Click for large image | Figure 1. Retrospective cohort study design. H: hours; IH: initially hypotensive; INT: initially normotensive; OSH: outside hospital; SBP: systolic blood pressure; MAP: mean arterial pressure. |
The remaining septic patients were identified as either cases of initial hypotension (IH) or initial normotension (INT). Patients with IH had systolic blood pressure (SBP) ≤ 90 mm Hg or mean arterial pressure (MAP) ≤ 65 mm Hg at the time of presentation. INT subjects presented with SBP > 90 mm Hg or MAP > 65 mm Hg but then developed hypotension in the first 24 h after hospitalization as defined by IH blood pressure parameters, or had a decrease in SBP more than 40 mm Hg from baseline, as consistent with Society of Critical Care Medicine/American College of Chest Physicians criteria for septic shock [1, 13-15].
We collected demographic data, chronic comorbidities, home medications, reason for hospital admission, length of stay, survival, laboratory data, and timing of sepsis treatment from their EMR. Respiratory failure was defined as requiring ventilator use; kidney failure was defined by a creatinine increase 1.5-fold from baseline; liver failure was defined by a bilirubin level greater than 1.2 mg/dL.
Statistical analysis
SPSS 21 (IBM, Armonk, NY) was used for database management and statistics. Data were analyzed by Student’s t-test and Pearson Chi-square test where appropriate. A two-sided P-value less than 0.05 was considered significant. We used logistic regression to examine clinical biomarkers that were predictive of the dichotomous outcome of being INT or IH. Variables identified as potential confounders and those with a P-value ≤ 0.05 in univariate analysis were included in the multivariate logistic regression model. Over-fitting was limited by excluding variables with inadequate prevalence and assessed by the Hosmer-Lemeshow goodness-of-fit. The model discrimination was evaluated using the receiver operating characteristic area under the curve (ROC-AUC).
| Results | ▴Top |
Demographic characteristics of cohort
Retrospective case cohort design of patients with sepsis in 2011 - 2012 based on inclusion/exclusion criteria is shown in Figure 1. Among a total of 542 patients who were admitted for sepsis, we identified 52 (9.6%) patients with IH and 62 (11.4%) patients with INT. Demographics of the INT and IH groups are described in Table 1.
 Click to view | Table 1. Demographics |
The two groups were not significantly different in age, gender, days of illness prior to presentation, or pre-hospitalization setting. They were significantly different in regards to race and BMI. INT patients were more likely to be Caucasian and had higher BMI. INT patients were also more likely to be on alpha-blockers and chronic steroids. They were also not significantly different in comorbidities, or most home medications (Table 2).
 Click to view | Table 2. Past Medical History |
Clinical presentation and source of infection
At presentation, mean heart rate and temperature were significantly higher in the INT group (Table 3).
 Click to view | Table 3. Clinical Parameters at Presentation |
The average blood pressure of the IH group was significantly lower (81/46, MAP: 57 mm Hg), compared to the INT group (121/71, MAP: 88 mm Hg). Over the next 24 h, laboratory values were obtained and the most abnormal values were recorded (Table 3). Patients in IH and INT groups had similar white blood cell counts, hemodynamic stability, and serum sodium values. The IH group had significantly higher levels of serum creatinine compared to INT, but this may be a reflection of the higher prevalence of end-stage renal disease (ESRD) in the IH group compared to INT (Table 2) as incidence of acute renal failure was not different. Mean bicarbonate serum level was also higher in INT patients, although both expressed average levels within the normal range (Table 3). The INT patients were also more likely to be septic from pneumonia compared to other sources of infection, whereas IH patients more likely had positive, gram positive cultures from specimens other than blood or urine (Table 4).
 Click to view | Table 4. Sepsis Source and Treatment |
Treatment and measures of outcome
INT and IH groups did not differ in treatment protocols for their sepsis (Table 4). Of interest, both groups received antibiotics within 3 h, but INT patients developed hypotension to an average of 79/48 mm Hg (MAP: 59 mm Hg) at 3 h and 18 min. INT and IH patients also received similar fluid volumes over the first 24 h, and had similar vasopressor and ventilator requirements. Other measures of the severity of illness were studied in the population (Table 5). The INT and IH groups had similar end-organ dysfunction, length of stay, and mortality at 28 days or 1 year. INT patients had a mean APACHE II score of 18, compared to IH patients with mean 20 (P = 0.07).
 Click to view | Table 5. Indices of Severity of Illness |
Association between development of INT and clinical biomarkers
A logistic regression model to predict INT was created and adjusted for race, source of infection, BMI, and age (Table 6). Source of sepsis was coded as a dichotomous variable of pneumonia versus other sources to prevent over-fitting. Potential biomarkers were individually tested as crude variables for ability to predict INT as an outcome, and then incorporated into a final multivariable model in stepwise regression.
 Click to view | Table 6. Logistic Regression Model Predictive of INT |
Outpatient alpha-blocker use was very predictive of being in the INT group and increased odds 302% (Table 6). An increase of 1 °F in temperature increased odds by 36% of being in the INT group. Of interest, race was also a significant predictor of INT. Caucasians were 323% more likely of presenting in the INT group. ROC curve analysis had AUC of 0.815 (95% CI: 0.736 - 0.894) and demonstrated good predictive ability (Supplementary Fig. 1, www.jocmr.org). The final model was robust and did not suffer from over-fitting, with Hosmer-Lemeshow Chi-square of 9.67 (P = 0.289). Sensitivity of the model was 75.0%, and specificity was 77.4%.
| Discussion | ▴Top |
The major findings of our retrospective study include that the presentation of INT is at least as common as IH subgroup. The INT patients differed from the IH patients in that they had increased sympathetic-adrenergic tone manifested by higher heart rate and body temperature, higher BMI, race, higher rate of pneumonia as the source of sepsis, and more frequent alpha-blocker and chronic steroid use as outpatient medications. We further identified that being Caucasian, on an alpha-blocker, and having a higher temperature increased the odds of declining further to septic shock.
Phenotyping the subgroup of patients that present with sepsis and further decline to shock has been the focus of recent studies. Similar to our findings, the proportion of septic patients who developed shock between 4 and 48 h after admission was more than half in this recently published work [11]. The great majority of these patients developed shock within 24 h, and pneumonia was a common source of infection. Variables associated with progression to shock included female gender, coronary artery disease, bandemia and elevated lactate [11].
Our study similarly sought to identify risk factors for progression to septic shock and suggest mechanisms that may contribute to vascular decompensation in INT patients. It could be argued that the INT patients were at an earlier point of their sepsis trajectory, and became acutely hypotensive as their disease progressed during the early hospital course. This hypothesis is supported by the findings that INT patients have fewer positive cultures and fewer isolates of gram-positive organisms than IH patients. However, number of days of illness prior to presentation did not differ significantly between the two groups. Additionally, INT patients’ APACHE II scores trended lower than those of the IH patients (P < 0.07), and would have differed more had APACHE II scores been taken at presentation before INT patients became hypotensive, rather than at 24 h. Moreover, less severity of illness at presentation would suggest better outcomes in the INT subgroup compared to the IH subgroup, given prior studies showing that early treatment with fluid resuscitation and antibiotics improve survival [7, 8-10].
Fluid resuscitation could have contributed to the transition from a hemodynamically compensated circulation in the INT patients to a full-blown septic shock state. Fluid resuscitation is a mainstay of treatment for septic shock to maintain pressure in the central circulation [9]. However, it does not treat the main pathophysiology of distributive shock and, when given excessively, it could potentially compromise the microcirculation due to worsening interstitial edema [16, 17]. The fluid expansion as supportive therapy (FEAST) trial questioned the role of fluid resuscitation in sepsis, showing higher mortality in fluid-resuscitated African children with sepsis than in non-resuscitated controls [6]. The post hoc analysis, based on presenting symptoms and predominant terminal clinical events, showed that cardiovascular collapse was the major cause of death after rapid fluid resuscitation rather than fluid overload [18]. A possible explanation is that rapid restoration of circulatory volume via fluid resuscitation has unintended consequences, including the interruption of the compensatory, sympathetic, and innate response to hypovolemia [17, 19]. This compensatory response distinguished the INT patients from the IH patients. The INT patients presented in a relatively hyperadrenergic state, maintaining blood pressure along with higher heart rate and body temperature. In this scenario, fluid bolus given early in sepsis management results in the rapid conversion from vasoconstriction to vasodilation by disseminating a localized and contained focus of cytokines from infected tissue to the systemic circulation. In this regard, we observed significantly more instances of pneumonia in the INT subgroup compared to the IH subgroup. Among the 20 patients with pneumonia overall, 15 were in the INT subgroup. This observation supports the hypothesis that the transition from normotension to septic shock may involve fluid-driven dissemination of cytokines, or other cell derived mediators, from a localized site of infection with a rich vascular supply, like the pulmonary microcirculation, to the systemic circulation. Although the INT subgroup did receive more fluid volume than the initial septic shock subgroup during the first 24 h (4.3 L vs. 3.9 L), the difference did not reach statistical significance.
An alternative explanation for hypotension during treatment of early sepsis may be the presence of a Jarisch-Herxheimer reaction following the administration of antibiotics [20, 21]. Time to initiation of first antibiotics was similar in both groups. Interestingly, antibiotic administration occurred before the recognition of hypotension in the INT subgroup (2 h 52 m vs. 3 h 18 m). Also, the INT group had relatively more gram-negative isolates than the septic shock subgroup. These observations suggest that antibiotics could contribute to the onset of septic shock via a Jarisch-Herxheimer reaction by lysing bacteria with the subsequent release of endotoxin. This question warrants further study.
We found two other differences between the INT and IH subgroups that may have affected their hemodynamic state at presentation. Although the Charlson comorbidity index was the same for both subgroups, INT patients were taking alpha-blockers as outpatients significantly more frequently than IH patients. Chronic alpha-blocker therapy may result in peripheral adrenergic receptor up-regulation and contribute to hyper-adrenergic compensation. Therefore, patients taking alpha-blockers may be more susceptible to acute decompensation after interruption of their hyper-adrenergic, compensatory response, as may occur after fluid resuscitation. Also, INT patients were more frequently on chronic steroid therapy prior to presentation, another factor that may have affected their ability to sustain a compensated hemodynamic state.
Limitations of our study include its retrospective design. Observed variables that are associated with the INT presentation cannot be regarded as causal. Also, the Veterans Affairs medical center has predominantly male patients with a potentially greater fraction of patients on alpha-blocker therapy, possibly limiting the generalizability of our findings. Finally, both INT and IH groups had similar mortality at 28 days and 1 year, similar organ failures, and similar ICU and hospital lengths of stay for both patient subgroups.
In summary, we found that the two presentations of septic shock, INT and IH, occur with equal frequency. Patient-specific variables may discriminate between these presentations. Although INT patients seem to have less severity of illness based on hemodynamic stability at presentation, we found no differences in important clinical outcomes between the two subgroups. However, because the INT group provides a point of origin, or time zero, for progression into septic shock, further study of this group may provide clarification of the pathophysiology and mechanisms of septic shock.
Key messages
In our population, 46% of patients who present with sepsis progress to septic shock.
Being Caucasian or on an alpha-blocker increased the odds of progressing to septic shock independently at least three-fold.
Each 1 °F increase above the mean of the cohort increased odds of progressing to shock 36%.
Timing of antibiotic initiation, amount of fluid resuscitation in first 24 h, vasopressor use, and mortality at 28 days did not increase odds of progression to shock.
Author Contributions
YIL, RLS and AN participated in study conception and design; YIL, RLS and AN were the primary investigators; YIL, RLS and AN were responsible for data collection; YIL, RLS and AN were responsible for data validation; YIL, EJC, SK and AN participated in data analysis; YIL, SK and AN undertook the statistical analysis. All authors participated in data interpretation, writing and revision of the report and approval of the final version. No individuals contributing to data collection, analysis, writing or editing assistance, and review of manuscript have been omitted.
Guarantor Statement
AN has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects.
The funding agencies did not participate in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Funding
NHLBI R01HL119326 and K23HL084191/S1, Saperstein Scholar Award and a Clinical and Translational Science Institute Pilot Project supported in part by grant UL1TR000038 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Conflicts of Interest
No conflicts of interest exist for any of the authors.
Abbreviations
DM: diabetes mellitus; ESRD: end-stage renal disease; EMR: electronic medical record; FEAST: fluid expansion as supportive therapy; IH: initial hypotension; INT: initial normotension; MAP: mean arterial pressure; SBP: systolic blood pressure; VA: veterans administration
| References | ▴Top |
- Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481-1483.
doi pubmed - Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138-150.
doi pubmed - Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554.
doi pubmed - Marik PE. Early management of severe sepsis: concepts and controversies. Chest. 2014;145(6):1407-1418.
doi pubmed - Rivers EP, Jaehne AK, Nguyen HB, Papamatheakis DG, Singer D, Yang JJ, Brown S, et al. Early biomarker activity in severe sepsis and septic shock and a contemporary review of immunotherapy trials: not a time to give up, but to give it earlier. Shock. 2013;39(2):127-137.
doi - Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483-2495.
doi pubmed - Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045-1053.
doi pubmed - Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596.
doi pubmed - Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
doi pubmed - Kumar A, Haery C, Paladugu B, Symeoneides S, Taiberg L, Osman J, Trenholme G, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193(2):251-258.
doi pubmed - Capp R, Horton CL, Takhar SS, Ginde AA, Peak DA, Zane R, Marill KA. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med. 2015;43(5):983-988.
doi pubmed - Bernardin G, Pradier C, Tiger F, Deloffre P, Mattei M. Blood pressure and arterial lactate level are early indicators of short-term survival in human septic shock. Intensive Care Med. 1996;22(1):17-25.
doi pubmed - Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644-1655.
doi pubmed - Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
doi pubmed - Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637.
doi pubmed - Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12(4):169.
doi pubmed - Elbers PW, Ince C. Mechanisms of critical illness - classifying microcirculatory flow abnormalities in distributive shock. Crit Care. 2006;10(4):221.
doi pubmed - Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68.
doi pubmed - Myburgh J, Finfer S. Causes of death after fluid bolus resuscitation: new insights from FEAST. BMC Med. 2013;11:67.
pubmed - Griffin GE. Cytokines involved in human septic shock - the model of the Jarisch-Herxheimer reaction. J Antimicrob Chemother. 1998;41(Suppl A):25-29.
doi pubmed - Negussie Y, Remick DG, DeForge LE, Kunkel SL, Eynon A, Griffin GE. Detection of plasma tumor necrosis factor, interleukins 6, and 8 during the Jarisch-Herxheimer Reaction of relapsing fever. J Exp Med. 1992;175(5):1207-1212.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


