| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 3, March 2018, pages 196-201
Haptoglobin Phenotype Among Arab Patients With Mental Disorders
Zaher Armalya, c, Kamal Farhatb, Safa Kinaneha, Joseph Farahb
aDepartment of Nephrology, Nazareth Hospital-EMMS, Nazareth and the Galilee Faculty of Medicine-Bar Ilan University, Zafed, Israel
bDepartment of Psychiatric, Nazareth Hospital-EMMS, Nazareth and the Galilee Faculty of Medicine-Bar Ilan University, Zafed, Israel
cCorresponding Author: Zaher Armaly, Department of Nephrology, The Nazareth Hospital-EMMS, Nazareth 16100, Israel
Manuscript submitted November 22, 2017, accepted December 11, 2017
Short title: Hp phenotype and Mental Disorders
doi: https://doi.org/10.14740/jocmr3279w
| Abstract | ▴Top |
Background: Depression, schizophrenia and panic disorder are common mental disorders in the community and hospitalized patients. These mental disorders negatively affect life quality and even expectancy of life. Haptoglobin (Hp) phenotype (Hp 1-1, 1-2, or 2-2) is associated with risk for cardiovascular diseases, but its association with psychiatric disorders, a growing concern in the modern society, has not been studied thoroughly. The aim of the study was to examine whether Hp phenotype is associated with common mental disorders such as depression, schizophrenia, and panic disorder.
Methods: The study included 92 Arab patients with mental disorders, and among them 44 suffered from schizophrenia (mean age 39 ± 1.5 years), 17 from depression (mean age 44.5 ± 3.1 years), 31 from panic disorder (mean age of 44.9 ± 2.7 years), and 206 healthy Arab control subjects with a mean age of 42.6 ± 0.9 years. Beck’s depression inventory assessment and Hamilton depression scale were administered for depression and panic disorder diagnosis. Schizophrenia was evaluated with positive and negative affect schedule (Panas) test. All mental disorders were evaluated by clinical review. Blood analysis for Hp phenotype was performed. Diagnosis was made using the Diagnostic and Statistical Manual of Mental Disorders axis to correlate depression with Hp phenotype.
Results: In mentally healthy controls, 10.7% were Hp 1-1, 38.8% Hp 2-1, and 50.5% Hp 2-2. In patients with the studied psychiatric disorders, Hp phenotype was comparable to healthy subjects; 8.7% were Hp 1-1, 50% Hp 2-1, and 41.3% Hp 2-2. When Hp phenotyping was analyzed in the psychiatric subgroups, Hp 2-1 was more common among depressed and schizophrenic patients, as compared with healthy subjects (58.8% and 52.3% vs. 38.8%). In patients who suffer from panic disorder, Hp phenotype distribution was 6.5% Hp 1-1, 41.9% Hp 2-1, and 51.6% Hp 2-2, suggesting a lower prevalence among Hp 1-1 phenotype.
Conclusions: Arab patients who carry Hp 2-1 phenotype may be at risk to develop depression or schizophrenia more than the general healthy population. In contrast, Hp 1-1 subjects have a lower prevalence of panic disorder.
Keywords: Haptoglobin; Mental disorders; Depression; Schizophrenia; Panic disorder; Association
| Introduction | ▴Top |
The prevalence of mental disorders including depression, schizophrenia, panic disorder, and compulsive obsession is steadily rising in the Western world. Although strategies for treating these disorders improved in the last decade, little is known concerning the underlying mechanisms that increased risk of certain subjects to develop these mental disorders. Susceptibility to mental disorders has been attributed to several factors including genetic ones [1-4].
Haptoglobin (Hp) is an abundant serum protein for which there are two common alleles in human being, termed 1 and 2 [5]. The phenotype of an individual may therefore be described as being Hp 1-1 (homozygous for the Hp 1 allele), Hp 2-2 (homozygous for the Hp 2 allele) or Hp 2-1 (heterozygote). The frequencies of the two Hp alleles typing have revealed considerable geographic and ethnic impact. In Israel, the two alleles are in a balanced polymorphism with approximately 30-40% of the alleles being type 1 and 60-70% type 2 [6-11].
Normal healthy individuals produce substantial amounts of intravascular free hemoglobin (Hb) due to daily turnover of red blood cells. Free extracorpuscular Hb represents a highly potent source of oxidative tissue damage due to the heme iron within the Hb which can act as a Fenton reagent to produce the highly reactive hydroxyl radical [5]. Hp protein functions as the main line of defense against the oxidative effects of Hb [5]. It has been shown that the Hp 1-1 protein is superior to the Hp 2-2 protein in this antioxidant function [12, 13]. The Hp phenotype has been associated with cardiovascular complications in numerous studies [6]. For instance, Hp 2-2 increases the susceptibility to develop cardiovascular and renal diseases of diabetes mellitus five-fold as compared with Hp 1-1 subjects [6-8]. Unfortunately, the interaction between the Hp phenotype and mental disorders has not been studied thoroughly. This issue is of special interest since most recently a linkage between Hp phenotype and poor cognitive functioning in the elderly with type 2 diabetes has been reported [14].
| Materials and Methods | ▴Top |
The study was approved by the Nazareth Hospital EMMS Human Research Review Committee and carried out at Nazareth Hospital. All patients provided informed consent.
The demographic and laboratory data of the studied Arab patients from Northern Israel are listed in Table 1. The current study included three subgroups of total 92 psychiatric patients: 17 suffered from depression, 44 from schizophrenia, and 31 from panic disorder. Two hundred and six healthy subjects (n = 206) served as controls.
 Click to view | Table 1. Demographic Characteristics of Studied Healthy Subjects and Mentally Ill Patients |
Mental disorders characterization
The Hamilton depression scale was administrated, and a score of 18 or greater on Hamilton depression scale accompanied by the presence of significant neurovegetative changes was evaluated to confirm the diagnosis of depression and panic disorders. We also used the Beck’s depression inventory (BDI), a self-administration evaluation including 21 questions, which is known to be reliable and valid, as the main evaluation method in our study. A BDI score of 11 or greater indicates the presence of at least a moderate level of depression symptoms. Patients with a Hamilton score of 18 and/or BDI score of 11 underwent a structured clinical interview by a psychiatrist based on DSM-V criteria. Diagnosis was made to correlate mental disorder with Hp phenotype. Schizophrenia was diagnosed according to positive and negative affect schedule (Panas) test.
Hp phenotype analysis
Hp phenotypes were determined as described by Hochberg et al [15]. Briefly, serum (10 µL) that were obtained from blood samples of the depressed, schizophrenic and anxious patients, and healthy subjects were mixed with 2 µL of a 10% hemoglobin solution, and the samples were incubated for 5 min at room temperature to permit the Hp-Hb complexes to form. An equal volume of sample buffer containing 125 mmol/L Tris base (pH 6.8), 200 g/L glycerol, and 0.01 g/L bromphenol blue was added to each sample before electrophoresis. The Hp-Hb complex was resolved by polyacrylamide electrophoresis using a buffer containing 25 mmol/L Tris base and 192 mmol/L glycine. The stacking gel was 4% polyacrylamide (29:1 acrylamide-bis-acrylamide) in 125 mmol/L Tris base, pH 6.8, and the separating gel was 4.7% polyacrylamide (29:1 acrylamide-bis-acrylamide) in 360 mmol/L Tris base, pH 8.8. Electrophoresis was performed at a constant voltage of 120 V for 2 h. After the electrophoresis was completed, the Hp-Hb complexes were visualized by soaking the gel in freshly prepared staining solution. The staining solution contained 5 mL of 2 g/L 3,3′,5,5′-tetramethylbenzidine in methanol, 0.5 mL of dimethyl sulfoxide, 10 mL of a 50 mL/L solution of glacial acetic acid, 1 mL of a 10 g/L potassium ferricyanide solution, and 150 µL of 300 g/L solution of hydrogen peroxide. The bands corresponding to the Hp-Hb complex were readily visible within 15 min and are stable for more than 48 h. All gels were documented with photographs. Phenotypes Hp 1-1, Hp 2-2, and Hp 2-1 were distinguished by a characteristic pattern of bands representing the Hp-Hb (Fig. 1).
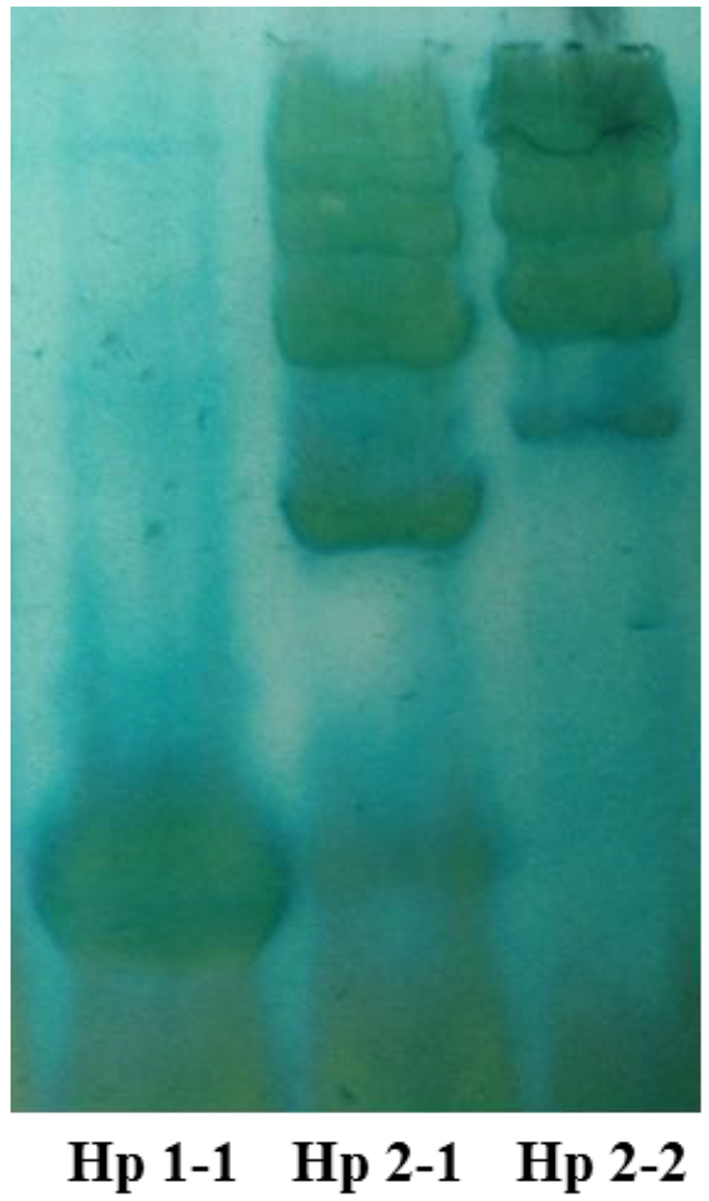 Click for large image | Figure 1. Representative profile of the three Hp phenotypes in plasma samples using native-PAGE. The pattern reveals heterogeneity in Hp 2-1 and Hp 2-2, but not in Hp 1-1. |
| Results | ▴Top |
Demographic characterization
The mean ages of the diseased groups and control subjects were comparable: schizophrenia (mean age 39 ± 1.5 years), depression (mean age 44.5 ± 3.1 years), and panic disorder (mean age of 44.9 ± 2.7 years), and healthy control subjects with a mean age of 42.6 ± 0.9 years (Table 1).
The prevalence of depression and panic disorder was higher among females than males, while schizophrenia was more common among males (Table 1). This is the reason why we choose to include more females in the control group than males.
Hp phenotype
This protocol was designed in order to examine whether Hp phenotype is associated with psychiatric disorders. The distribution of mental disorders among the studied 92 patients is described in Figure 2a. The prevalence of schizophrenia was 47.8%, depression 18.5%, and panic disorder 33.7%. Figure 2b depicts the distribution of Hp gene polymorphism among the studied psychiatric patients, as compared with the healthy controls. Hp 1-1 among healthy subjects was 10.7%, 38.8% Hp 2-1, and 50.5% Hp 2-2. About 9% of the psychiatric patients were Hp 1-1, comparable to healthy subjects. However, 50% of the psychiatric patients were Hp 2-1, and 41.3% Hp 2-2 (Fig. 2b).
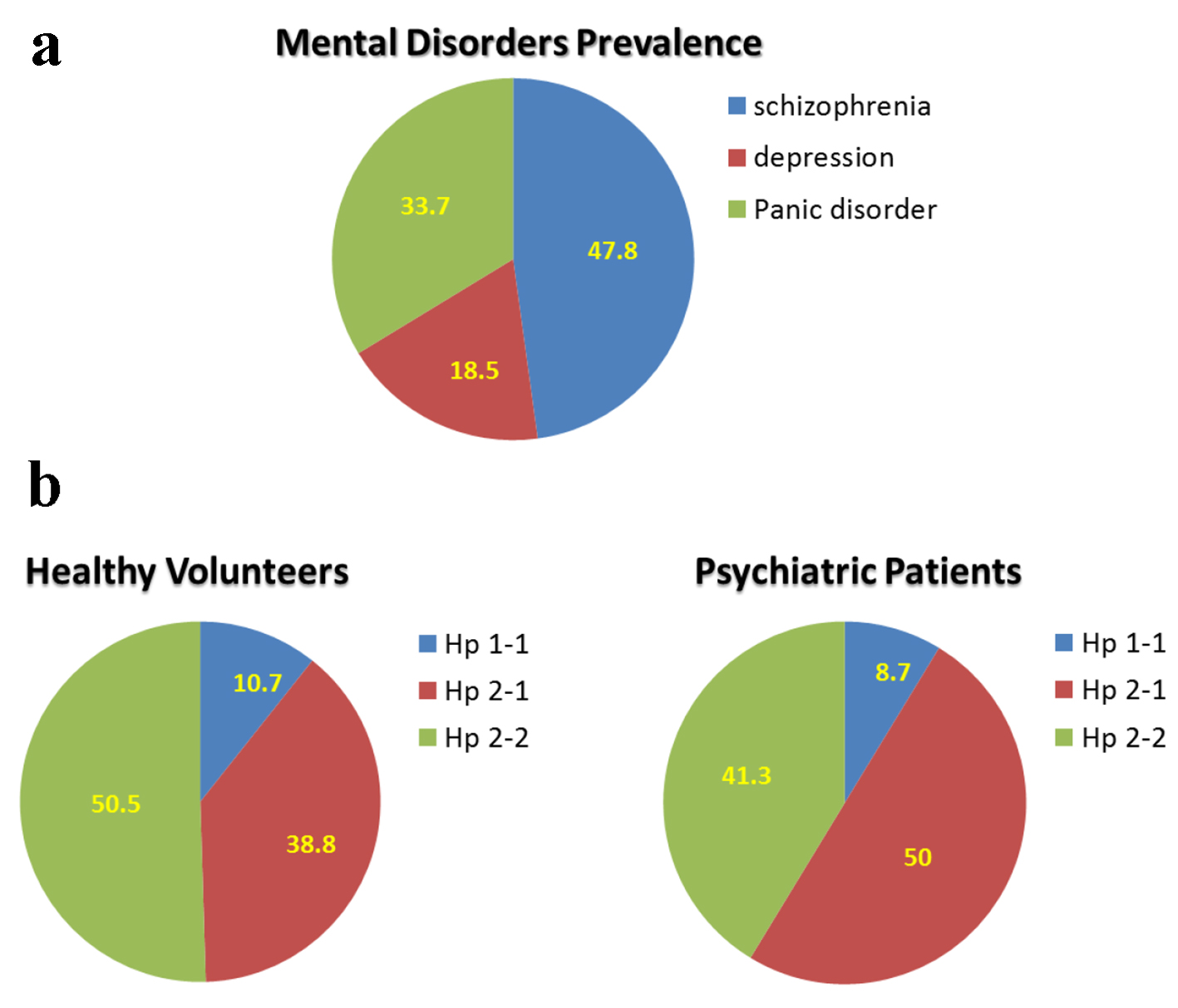 Click for large image | Figure 2. Mental disorder prevalence and distribution of haptoglobin phenotype among healthy subject and patients with mental illness: depression, schizophrenia, and panic disorder. |
When the psychiatric patients were divided into three subgroups (schizophrenia, depression, and panic disorder), a distinct Hp phenotype distribution was found among these subgroups of mental disorders (Fig. 3). Specifically, Hp 1-1 among depressed patients was 11.8%, 58.8% Hp 2-1, and 29.4% Hp 2-2. Among schizophrenic patients, Hp 1-1 phenotyping was 9.1%, 52.3% Hp 2-1, and 38.6% Hp 2-2. Finally, among patients who suffer from panic disorder, the distribution of Hp phenotype for Hp 1-1, Hp 2-1, and Hp 2-2 was 6.5%, 41.9%, and 51.6%, respectively. These results suggest that depression and schizophrenia, but not panic disorder, are more common among Hp 2-1 patients.
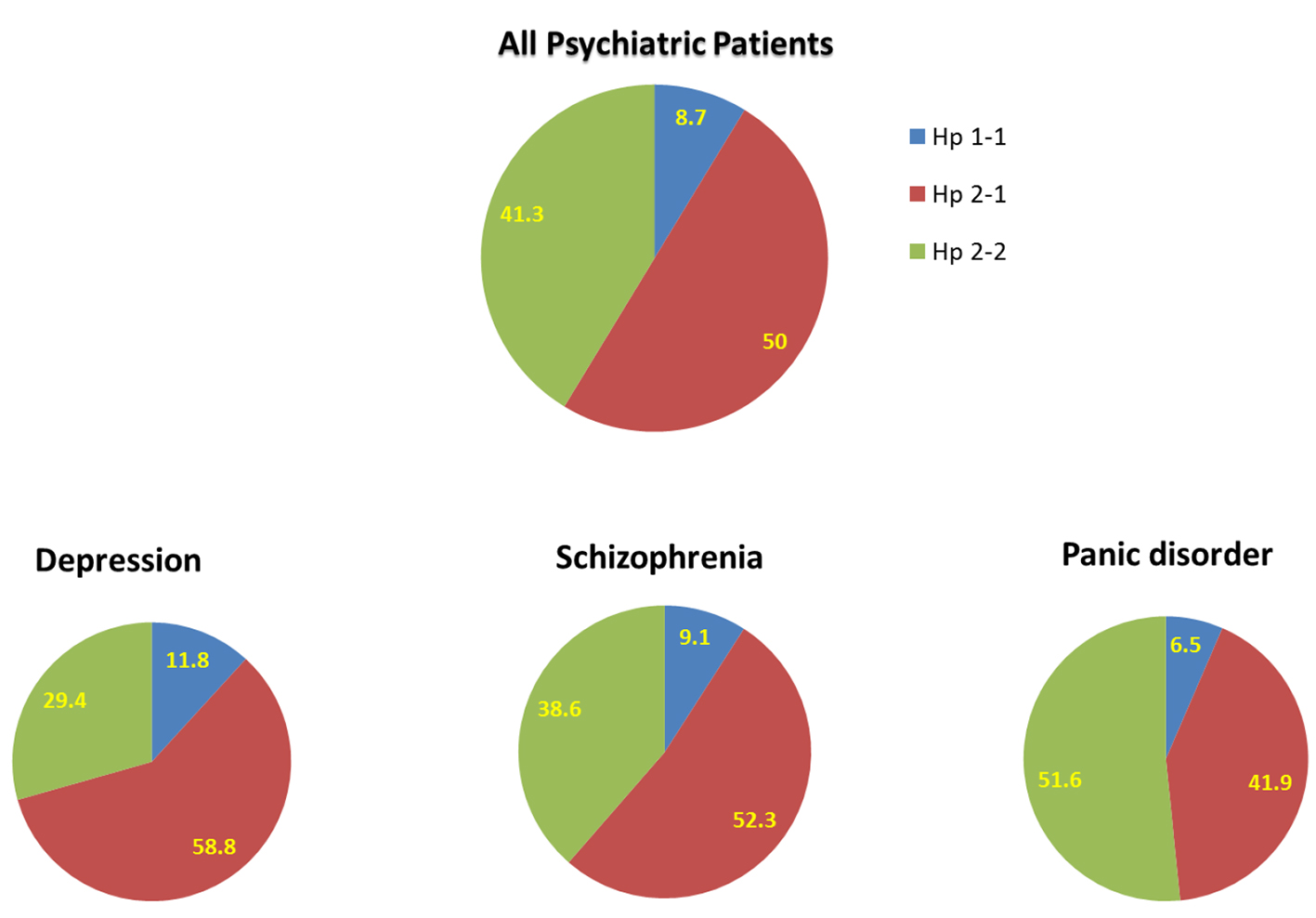 Click for large image | Figure 3. Distribution of haptoglobin phenotype among all patients with mental illness and subgroups of patients with depression, schizophrenia, and panic disorder. |
| Discussion | ▴Top |
Psychiatric disorders are very heterogeneous clinical problems with many different etiologies. By understanding the underlying causes of these diseases, progress towards effective treatment can be made. Studies have demonstrated an association between Hp phenotype and certain mental disorders such as schizophrenia, but not depression and panic disorder. Therefore, the current study extends our knowledge in this regard, where we demonstrate distinct distribution of Hp phenotype among psychiatric patients. Specifically, Hp 2-1 was more common among depressed and schizophrenic patients, as compared with healthy subjects (58.8% and 52.3% vs. 38.8%). In patients who suffer from panic disorder, Hp phenotype distribution was 6.5% Hp 1-1, 41.9% Hp 2-1, and 51.6% Hp 2-2, suggesting a lower prevalence of the disease among Hp 1-1 phenotype. Thus, one may conclude that patients who carry Hp 2-1 phenotype may be at risk to develop depression or schizophrenia more than the general healthy population. In contrast, Hp 1-1 subjects have a lower prevalence of panic disorder.
Hp is an abundant Hb-binding protein present in the plasma [5, 12, 13]. There are two classes of functional alleles Hp 1-1 and 2-2. Hp binds to free Hb released from blood cells as part of red cell turnover, thus inhibiting the oxidative tissue damage resulting from free Hb through heme iron [12, 16]. Several studies have shown that the severity of oxidative stress-induced cardiovascular complications in various disease states is affected by Hp phenotype [6-11]. Hp alleles differ in their ability to clear free Hb from the plasma where Hp (1-1)-Hb complexes are cleared more efficiently from the plasma than Hp (2-2)-Hb complexes [5, 12, 16], thus subjects with Hp 2-2 are more prone to oxidative stress [17]. Although the association of Hp phenotype with risk for cardiovascular diseases and renal diseases is well established, its connection with psychiatric disorders, a growing concern in the modern society, has not been studied thoroughly. Most of the scarce studies in this regard, addressed the association of Hp and schizophrenia or depression and the results of these studies have been inconclusive.
Concerning schizophrenia, it is known that schizophrenia is accompanied by both an altered expression of Hp protein and a different phenotype distribution of Hp gene, suggesting that Hp is associated with schizophrenia. Therefore, Hp and transferrin types were studied in schizophrenic patients and control subjects by several groups. In agreement with our results, it has been demonstrated that Hp system displays a significant departure from the Hardy-Weinberg equilibrium with an excess of heterozygotes among the schizophrenic patients [18]. Likewise, the distribution of Hp types in the schizophrenic patients was significantly different from that in the controls. In contrast to Hp, there was no significant difference between patients and controls with respect to transferrin types [18]. In line with these findings, a study on 98 Northwestern Italian schizophrenic patients revealed that the allele frequency of the Hp phenotypes in schizophrenia, i.e. Hp 1-1 (9.2%), Hp 2-1 (38.8%) and Hp 2-2 (52.0%), was significantly different from that in the Northwestern Italian population, i.e. Hp 1-1 (17.0%), Hp 2-1 (51.3%) and Hp 2-2 (38.5%) [19]. The frequency of the Hp-2 gene was significantly higher in schizophrenic patients (71.7%) as compared with the observed frequency in the Northwestern Italian population (62.5%). The authors concluded that, schizophrenia is accompanied by an altered distribution of the Hp genotypes and phenotypes, suggesting that genetic variation on chromosome 16 may be associated with schizophrenia. Similarly, in genetic association study, Wan et al [20] found significant associations existing between schizophrenia and Hp polymorphisms, Hp 1/2 and rs2070937 variants. The results from proteomic and genomic aspects both indicate that acute phase reaction is likely to be an etiological agent in the pathophysiology of schizophrenia, but not just an accompanying symptom. The positive acute phase proteins (APPs) are schizophrenic related proteins, with the highly concordant results on four positive APPs. In contrast to these reports, a study in Chinese patients revealed no such association between Hp or transferrin polymorphisms and schizophrenia [18, 21]. Moreover, no reliable differences in the distribution of Hp phenotypes were found between schizophrenic patients and healthy Russian persons [22]. However, hereditary types of Hp turned to be markers of the course and the prognosis of schizophrenia.
Regardless the Hp phenotype, a recent study by Sone et al [23] demonstrated that mean serum Hp levels were significantly higher in patients with Alzheimer disease compared with healthy controls, but comparable with those of Parkinson disease group. Interestingly, these authors found a significant positive association between the serum Hp level and the severity of cognitive impairment, as measured using several neuropsychological tests, in the patients with AD. It would have been of interest to determine Hp phenotype in these patients besides measuring the circulatory levels of the peptide.
Pursuing the hypothesis that certain unipolar and bipolar depression may be genetically related, we and others analyzed Hp phenotype in depressed patients. Moreover, several studies have shown that major depression is accompanied by significantly increased plasma levels of positive acute-phase proteins such as Hp [24]. Thus, the current study revealed a higher prevalence of depression among Hp 2-1. In line with our finding, a significant higher frequency of the HP*1 allele has been detected in patients with unipolar major depression [24]. An increase of HP*1 allele frequency was found in the subgroup of patients with family history of exclusively unipolar disorder (70% in patients vs. 38% in controls, Chi-square = 8.34, P = 0.004). The relative risk for the HP*1 carriers in this subgroup was 3.8 (Chi-square = 7.29, P = 0.007). In this context, however, by applying associative studies in unipolar depression, other researchers [25] failed to find an association between the Hp-2 allele and depression in elderly patients with unipolar depression. Collectively, these findings suggest a genetic and etiological heterogeneity in the bipolar disorder, but not in all studies and ethnic groups.
Finally, panic disorder is a common illness that can have adverse consequences, including enhanced morbidity and mortality rates. Based on family and twin studies, it is widely accepted that panic disorder is a genetic disorder and transmission patterns within families support the hypothesis of a disease gene predisposing to the condition [26]. Therefore, efforts were invested to identify genes underlying familial panic disorders through linkage analysis. A preliminary linkage analysis of panic disorder at the University of Iowa found suggestive but inconclusive evidence of linkage to the alpha-Hp locus on chromosome 16q22 [26]. Our study show patients who carry Hp 1-1 phenotype have a lower prevalence of panic disorder.
In summary, our results clearly show that schizophrenia and depression are associated with Hp 2-1 while panic disorder is less connected to Hp 1-1 phenotype. It is an associative study, and additional studies are needed to establish cause and effect relationship between Hp phenotype and mental illnesses. However, taking into account that Hp is characterized by a molecular variation with three known phenotypes, i.e. Hp 1-1, Hp 2-1 and Hp 2-2, where the latter is associated with poorer cardiovascular and diabetic prognosis, it is appealing that such relation may exit also in major mental illnesses. This notion is further supported by the fact that Hp 2-2 phenotype is associated with an increased incidence of micro- and macro-vascular complications in type 2 diabetic subjects [6, 27], which may affect the cerebrovascular diseases.
Yet, it should be emphasized that the current study has few limitations, including small sample size, and Hp phenotype rather than genotype determination.
Conflict of Interest
All authors declare that there is no conflict of interest regarding the publication of this paper.
| References | ▴Top |
- Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3(2):171-178.
doi - Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388(10043):505-517.
doi - Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustun TB, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18(1):23-33.
doi pubmed - Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291(21):2581-2590.
doi pubmed - Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12(2):293-304.
doi pubmed - Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, Howard BV, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol. 2002;40(11):1984-1990.
doi - Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care. 2003;26(9):2628-2631.
doi pubmed - Suleiman M, Aronson D, Asleh R, Kapeliovich MR, Roguin A, Meisel SR, Shochat M, et al. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;54(9):2802-2806.
doi pubmed - Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28(2):341-347.
doi pubmed - Levy AP, Gerstein HC, Miller-Lotan R, Ratner R, McQueen M, Lonn E, Pogue J. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care. 2004;27(11):2767.
doi pubmed - Blum S, Vardi M, Brown JB, Russell A, Milman U, Shapira C, Levy NS, et al. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics. 2010;11(5):675-684.
doi pubmed - Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004;43(13):3899-3906.
doi pubmed - Mehta NU, Reddy ST. Role of hemoglobin/heme scavenger protein hemopexin in atherosclerosis and inflammatory diseases. Curr Opin Lipidol. 2015;26(5):384-387.
doi pubmed - Ravona-Springer R, Heymann A, Schmeidler J, Guerrero-Berroa E, Sano M, Preiss R, Koifman K, et al. Haptoglobin 1-1 genotype is associated with poorer cognitive functioning in the elderly with type 2 diabetes. Diabetes Care. 2013;36(10):3139-3145.
doi pubmed - Hochberg I, Roguin A, Nikolsky E, Chanderashekhar PV, Cohen S, Levy AP. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis. 2002;161(2):441-446.
doi - Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12:189-261, 453-184.
- Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, Rock W, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes. 2008;57(10):2794-2800.
doi pubmed - Bamm VV, Geist AM, Harauz G. Correlation of geographic distributions of haptoglobin alleles with prevalence of multiple sclerosis (MS) - a narrative literature review. Metab Brain Dis. 2017;32(1):19-34.
doi pubmed - Maes M, Delanghe J, Bocchio Chiavetto L, Bignotti S, Tura GB, Pioli R, Zanardini R, et al. Haptoglobin polymorphism and schizophrenia: genetic variation on chromosome 16. Psychiatry Res. 2001;104(1):1-9.
doi - Wan C, La Y, Zhu H, Yang Y, Jiang L, Chen Y, Feng G, et al. Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids. 2007;32(1):101-108.
doi pubmed - Malik S, Fu L, Juras DJ, Karmali M, Wong BY, Gozdzik A, Cole DE. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci. 2013;50(1):1-22.
doi pubmed - Sorokina TT. [Hereditary polymorphism in the haptoglobin system of schizophrenic patients and healthy subjects]. Genetika. 1976;12(9):164-165.
pubmed - Song IU, Kim YD, Chung SW, Cho HJ. Association between serum haptoglobin and the pathogenesis of Alzheimer’s disease. Intern Med. 2015;54(5):453-457.
doi pubmed - Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr. 2016;21(2):184-198.
doi pubmed - Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol. 2007;29(2):92-110.
doi pubmed - Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. Guilford Press; 2007.
- Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes. 2009;58(12):2904-2909.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


