| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 12, December 2016, pages 908-915
Antioxidant and Renoprotective Effects of Mushroom Extract: Implication in Prevention of Nephrolithiasis
Ariel Schulmana, Matthew Chaimowitza, Muhammad Choudhurya, Majid Eshghia, Sensuke Konnoa, b
aDepartment of Urology, New York Medical College, Valhalla, NY, USA
bCorresponding Author: Sensuke Konno, Department of Urology, New York Medical College, BSB Room A03, Valhalla, NY 10595, USA
Manuscript accepted for publication October 05, 2016
Short title: Mushroom Extract With Antioxidant Activity
doi: http://dx.doi.org/10.14740/jocmr2781w
| Abstract | ▴Top |
Background: The pathogenesis of nephrolithiasis (kidney stone) remains elusive, while several therapeutic options are available but not effective as we expected. Accumulating data yet suggest that oxidative stress (generation of oxygen free radicals) may play a primary role in its occurrence. Particularly, calcium oxalate (CaOx) is a key element in the most common form (> 75%) of kidney stones, and its crystal form known as CaOx monohydrate (COM) has been shown to exert oxidative stress, facilitating CaOx stone formation. Hence, diminishing oxidative stress with certain antioxidants could be a potential strategic approach. We are interested in a bioactive extract of Poria mushroom, PE, which has been shown to have antioxidant and renoprotective activities. Accordingly, we investigated if PE might have antioxidant activity that would have implication in prevention of kidney stone formation.
Methods: Renal epithelial LLC-PK1 cells were employed and exposed to COM or hydrogen peroxide (H2O2) as a positive control capable of exerting oxidative stress. Possible antioxidant and protective effects of PE against oxidative stress (exerted by COM or H2O2) were assessed by cell viability test and lipid peroxidation (LPO) assay. To explore its protective mechanism, two glycolytic parameters, hexokinase (HK) activity and ATP synthesis, were examined and cell cycle analysis was also performed.
Results: Both H2O2 and COM led to a significant (P < 0.05) reduction in cell viability, accompanied by severe oxidative stress assessed by LPO assay. Such oxidative stress also caused the significant decline in HK activity and cellular ATP level, indicating the inhibition of glycolysis. Cell cycle analysis further indicated that oxidative stress interfered with cell cycle, inducing a G1 cell cycle arrest that presumably results in the cessation of cell proliferation. However, PE was capable of significantly preventing or diminishing all these cellular effects mediated through oxidative stress (exerted by H2O2 and COM).
Conclusions: The present study shows that the mushroom extract PE appears to have antioxidant and renoprotective effects against oxidative stress exerted by COM in renal cells. Therefore, PE with antioxidant activity is considered a promising natural agent that may have clinical implications in prevention of nephrolithiasis primarily induced by oxidative stress.
Keywords: Mushroom extract; Antioxidant; Oxidative stress; Nephrolithiasis
| Introduction | ▴Top |
Oxidative stress or generation of oxygen free radicals during aerobic metabolic process [1] is one of major causes for cellular damage/dysfunction. Accumulating data suggest that such oxidative stress could be a primary cause of various renal cell injury/damage as well [1, 2]. Those include acute renal injuries by ischemia/reperfusion, nephrotoxic agents, or even by extracorporeal shock wave lithotripsy that is one of therapeutic procedures for removal of kidney stones [3, 4]. Thus, many renal diseases/disorders due to real cell injury are somehow linked to the certain degrees of oxidative stress.
In fact, renal cell injury is also considered a major risk factor for crystal deposition in the kidneys (nephrolithiasis) [5, 6]. In particular, calcium oxalate (CaOx) is a key element in the most common form (> 75%) of kidney stones [7] but a binding of CaOx crystals to the renal tubular epithelium is believed to be required for the ultimate stone development [8]. In addition, renal cell injury appears to be pre-requisite for such CaOx binding to the urothelium [9, 10]. In other words, no (CaOx) stone development is possible unless CaOx crystals bind to the renal tubular cells that were already injured or damaged. Actually, crystallization of CaOx forms or becomes “CaOx monohydrate (COM)” [5, 10], which has been shown to facilitate a stone formation through oxidative stress.
If oxidative stress were indeed the critical factor for such kidney stone formation, it is plausible that antioxidants might be able to prevent or reduce the incidence of nephrolithiasis. In fact, antioxidant enzymes such as catalase and superoxide dismutase and chemical (non-enzymatic) antioxidant, vitamin E, have been shown to effectively reduce oxidative stress, resulting in decreased renal cell injury and crystal deposition in the kidneys [11]. Our previous study also demonstrated that a potent antioxidant, N-acetylcysteine (NAC), was capable of significantly (> 70%) preventing and reducing chemically induced CaOx crystal formation in rats [12].
Besides NAC, we were also interested in studying a natural agent that was capable of preventing kidney stone formation and came across a bioactive mushroom extract called “PE” isolated from Poria mushroom [13]. This mushroom is not a new mushroom but is one of well-established medicinal mushrooms, which has been used in traditional Chinese medicine (TCM) for 2,000 years. The major chemical constituents of PE such as triterpenes, polysaccharides, and steroids have been also identified [13, 14]. A number of studies revealed that PE had renoprotective, antitumor, immunomodulatory, antibacterial, antioxidant, anti-hyperglycemic, anti-inflammatory, diuretic effects and so forth [15-21]. We are particularly interested in its renoprotective and antioxidant activities, which may help reduce the incidence of kidney stones induced by oxidative stress.
Accordingly, we investigated if PE with possible antioxidant activity would protect renal cells from oxidative stress exerted by COM. Additionally, we also explored the protective mechanism of PE against such oxidative stress. More details are described and the significant findings are also discussed herein.
| Materials and Methods | ▴Top |
Cell culture
The renal tubular epithelial LLC-PK1 cells (American Type Culture Collection, Manassas, VA) were employed as our experimental model in vitro. They were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 μg/mL). Cells were incubated at 37 °C in a 5% CO2 and 95% air. Routinely, culture medium was changed every 3 - 4 days and the passage of cells was performed weekly.
Experiments using COM and PE
Crystals of COM were purchased (Sigma-Aldrich, St. Louis, MO) and suspended with phosphate-buffered saline (PBS) to obtain a uniform slurry COM solution (20 mg/mL). PE (Poria mushroom extract) was a gift from the manufacturer (Mushroom Wisdom, Inc., East Rutherford, NJ) and dissolved in H2O to prepare a PE stock (25 mg/mL). Hydrogen peroxide (H2O2) was used as a positive control for exerting oxidative stress in this study. For experiments, cells (2 × 105 cells/mL) were first seeded in the six-well plates or T-75 flasks for 24 h and treated with the specified concentrations of H2O2, COM, PE or their combinations for another 24 h (specific experimental conditions were also described in Results). Cell viability was then determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay described below.
Cell viability test (MTT assay)
Cell viability was determined by MTT assay following the vendor’s protocol (Sigma-Aldrich, St. Louis, MO). This will indicate what % of cells is yet viable under oxidative stress (exerted by H2O2 or COM). MTT reagent (1 mg/mL) was added to the six-well plate that was then incubated for 3 h at 37 °C. After MTT was discarded, dimethyl sulfoxide (DMSO) was added to the plate and the absorbance of formazan solution (purple color) was read in a microplate reader. Cell viability was expressed by the % of sample readings relative to the controls (100%).
Lipid peroxidation (LPO) assay
The severity of oxidative stress was assessed by the LPO assay, by measuring the amount of malondialdehyde (MDA) formed in the plasma membrane, due to oxidative stress [22]. As MDA is an end product from peroxidation of polyunsaturated fatty acids, the severity of oxidative stress can be indicated as: the more MDA formed, the greater oxidative stress. The LPO colorimetric assay kit (Abcam, Cambridge, MA) was used and the procedures were described in the vendor’s protocol. The amount of MDA formed (in each sample) was then expressed by μM determined from the MDA standards.
Assays for hexokinase (HK) activity
HK activity was determined by the HK Colorimetric Assay Kit (Biovision, Milpitas, CA) following the manufacturer’s protocol with minor modifications. Control or agents-treated cells were lysed in HK buffer provided and supernatant (cell lysates) was obtained by centrifugation. The NADH standards and cell lysates (20 μg per sample) were prepared in the 96-well plate and the reaction was started by the addition of reaction mix (containing substrate). Immediately the plate was placed in a microplate reader and the absorbance changes with time were monitored at 450 nm for 20 min with 5-min intervals. All readings were calculated and normalized and then HK activity was expressed by the % of sample activity relative to the controls (100%).
Determination of cellular ATP level
The cellular ATP level was determined using the ATP Colorimetric Assay Kit (Biovision, Milpitas, CA) following the manufacturer’s protocol. The reaction was started by the addition of cell lysates (20 μL) to the reaction mixture in the 96-well plate. All samples including ATP standards (without cell lysates) were read at 570 nm on a microplate reader and ATP content in sample was calculated using ATP standards. The cellular ATP level was expressed by the % of sample readings relative to the controls (100%).
Cell cycle analysis
A BD FACscan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ), equipped with a double discrimination module, was employed for cell cycle analysis. Cells (about 1 × 106 cells) were resuspended in propidium iodide solution and incubated for 1 h at room temperature. Approximately 10,000 nuclei from each sample were analyzed on a flow cytometer, and CellFit software was used to quantify cell cycle compartments to estimate the % of cells distributed in the different cell cycle phases.
Statistical analysis
All data were presented as mean ± standard deviation (SD), and statistical differences between groups were assessed with the unpaired Student’s t-test or one-way analysis of variance (ANOVA). Values of P < 0.05 are considered to indicate statistical significance.
| Results | ▴Top |
Effects of H2O2 or COM on LLC-PK1 cell viability
Cytotoxic effect of oxidative stress on renal epithelial LLC-PK1 cells was assessed. Cells were treated with H2O2 (0 - 100 μM), a positive mediator of oxidative stress, for 24 h and cell viability was determined. Such study showed that cell viability was significantly reduced to about 70%, 50%, and 20% with 30, 70, and 100 μM H2O2, respectively (Fig. 1a).
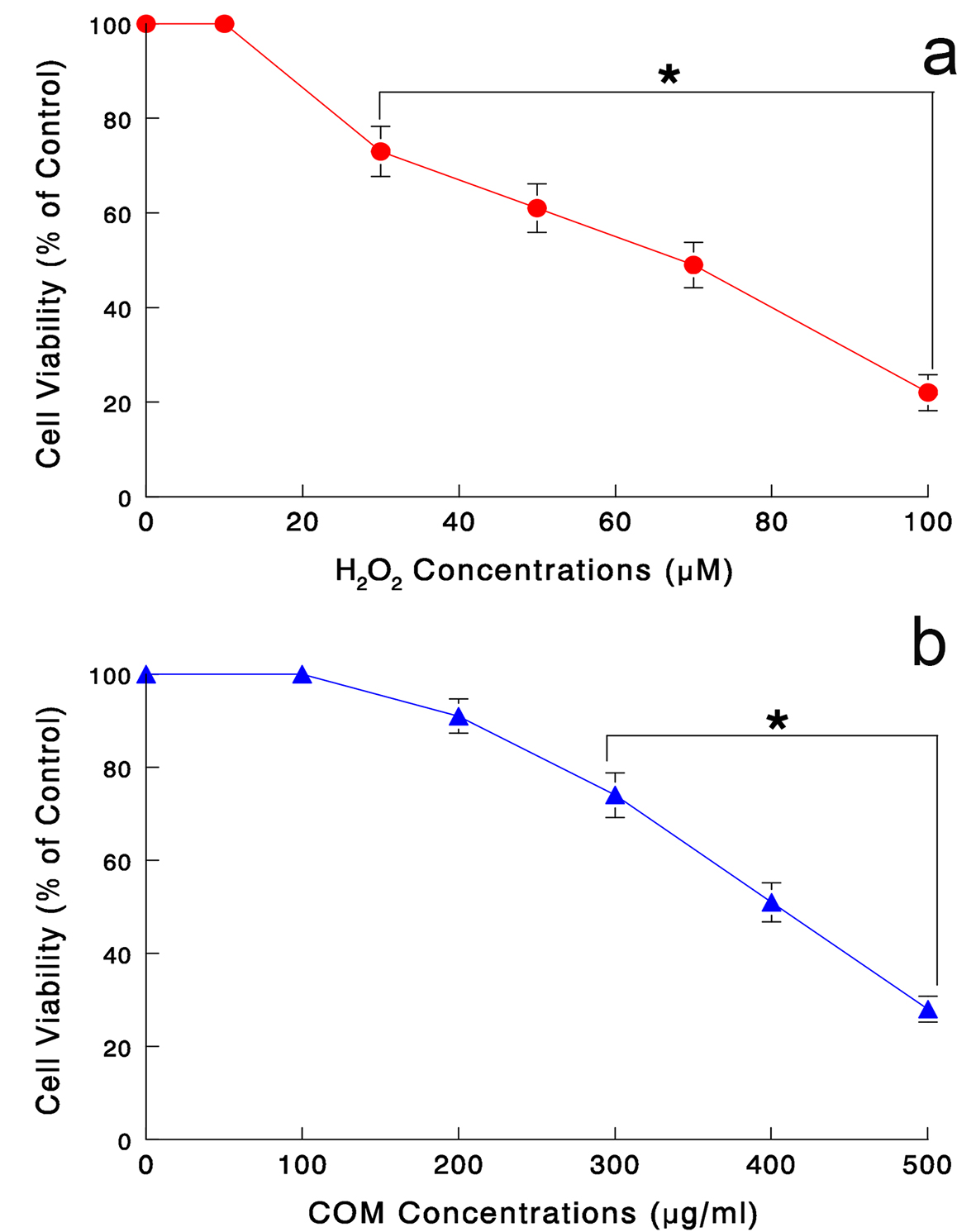 Click for large image | Figure 1. (a) Dose-dependent effects of H2O2 on LLC-PK1 cell viability. Cells were treated with varying concentrations of H2O2 (0 - 100 μM) for 24 h and cell viability was determined by MTT assay. All data are mean ± SD from three independent experiments (*P < 0.05). (b) Dose-dependent effects of COM on cell viability. Cells exposed to varying concentrations of COM (0 - 500 μg/mL) for 6 h were assayed for cell viability. The data are mean ± SD from three separate experiments (*P < 0.05). |
Similarly, cytotoxic effect of COM shown to exert oxidative stress [10] was also assessed. Our pilot study has indicated that 6-h exposure with varying concentrations of COM was sufficient to induce adverse effects on cell viability (data not shown). Cells were exposed to COM (0 - 500 μg/mL) for 6 h, followed by MTT assay. COM concentrations ≥ 300 μg/mL led to a significant cell viability reduction, which further declined to about 30% with 500 μg/mL (Fig. 1b).
Thus, these results suggest that both H2O2 and COM are indeed capable of reducing cell viability, presumably through oxidative stress. The estimated IC50 values for H2O2 and COM were 70 μM and 400 μg/mL, respectively (Fig. 1a, b). Hereafter, the rest of our study was carried out following the experimental conditions with “24-h H2O2 (70 μM)” and “6-h COM (400 μg/mL)”.
Antioxidant effect of PE on oxidative stress exerted by H2O2 or COM
To verify if H2O2 and COM exert oxidative stress, we directly assessed the severity of their oxidative stress by LPO assay. As damaging the plasma membrane by oxidative attack takes place at the early time, cells were exposed to H2O2 (70 μM), COM (400 μg/mL), or PE (50 μg/mL) alone and its combination with H2O2 or COM for 3 or 6 h. LPO assay showed that no apparent changes in the amount of MDA formed were seen in control or PE-exposed cells (Fig. 2). However, H2O2 and COM led to about 3.3-fold and 2.2-fold increase (compared to controls) in the MDA amounts at 6 h, respectively (Fig. 2). Such an MDA increase was yet significantly reduced by about 20% and 22% with PE in H2O2- and COM-exposed cells, respectively (Fig. 2). Therefore, both H2O2 and COM appear to exert severe oxidative stress on LLC-PK1 cells but PE with antioxidant activity is capable of effectively blocking such oxidative assault.
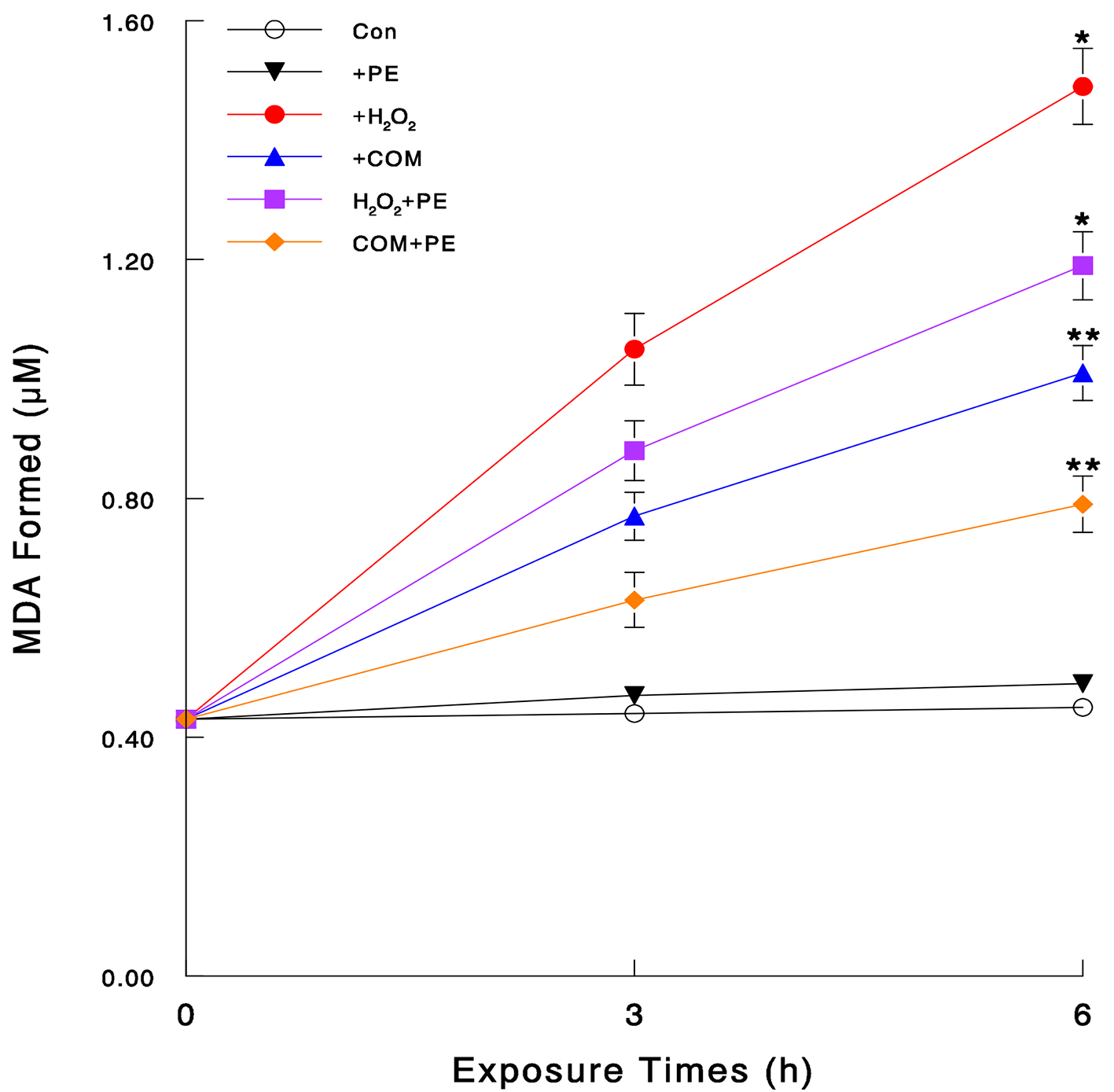 Click for large image | Figure 2. Severity of oxidative stress assessed by LPO assay. Cells were exposed to H2O2 (70 μM), COM (400 μg/mL), PE (50 μg/mL), or PE (50 μg/mL) combined with either H2O2 (70 μM) or COM (400 μg/mL), for 3 or 6 h. The amounts of MDA formed, assessed by LPO assay, were plotted against times. All data are mean ± SD from three separate experiments (*P < 0.03 and **P < 0.05 compared with controls). |
Renoprotective effect of PE against oxidative stress
We next examined if PE might also protect renal cells from oxidative attack. Cells were exposed to H2O2 (70 μM for 24 h), COM (400 μg/mL for 6 h), or PE (50 μg/mL for 24 h) alone and its combination with H2O2 or COM. MTT assay showed that both H2O2 and COM alone led to a significant (about 50%) reduction in cell viability, which yet went up to about 70% and 76% with PE in H2O2- and COM-exposed cells, respectively (Fig. 3). Thus, PE may effectively protect renal cells from oxidative stress, implying its renoprotective effect.
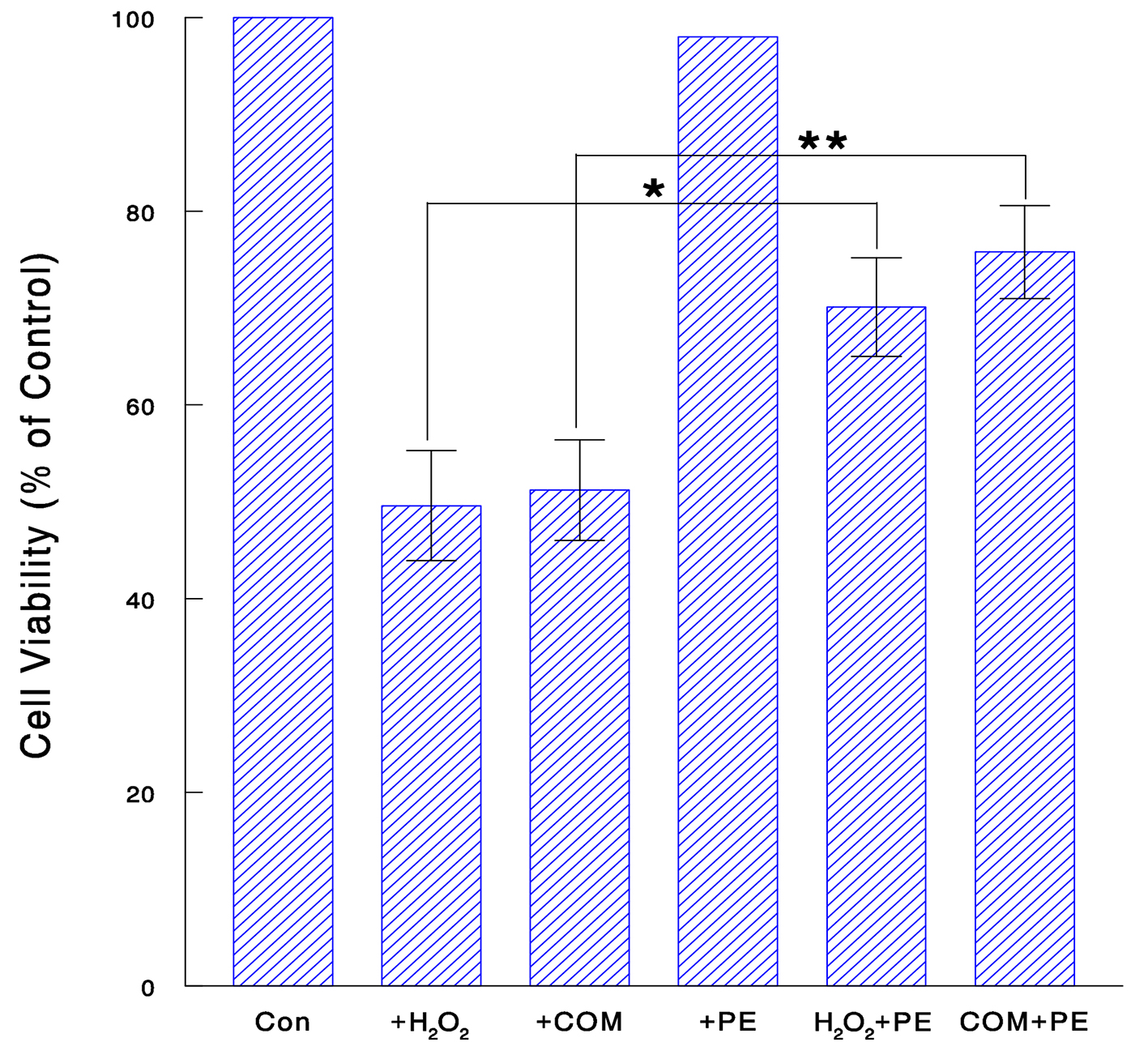 Click for large image | Figure 3. Renoprotective effect of PE against oxidative stress. Cells were treated with H2O2 (70 μM for 24 h), COM (400 μg/mL for 6 h), PE (50 μg/mL for 24 h), or PE combined with H2O2 (for 24 h) or COM (for 6 h). Cell viability was determined and the data are mean ± SD from three independent experiments (*P < 0.05; **P < 0.03). |
Effects of COM on glycolysis
Although we now know that COM could exert oxidative stress and reduce cell viability, it is yet uncertain “how” it is done. To address the relevance of this COM-induced cell viability reduction to nephrolithiasis, its cytotoxic mechanism was further explored. In particular, we examined the effects of COM on glycolysis, which is a major metabolic process for generating cellular energy (ATP) required for cellular activity, proliferation and survival [23, 24]. Two glycolytic parameters, HK activity and ATP synthesis, were examined to assess the status of glycolysis. Cells were exposed to COM (400 μg/mL) or PE (50 μg/mL) alone or their combination (COM + PE) for 6 h and assayed for HK activity and ATP synthesis. Compared to controls (100%), HK activity and ATP level declined to about 43% and 38% with COM, respectively (Fig. 4). This indicates that glycolysis is critically inhibited by COM, leading to cellular ATP depletion, followed by the growth cessation. However, PE significantly increased such COM-reduced HK activity and ATP level up to about 67% and 62%, respectively (Fig. 4). Therefore, PE may effectively prevent the glycolysis inhibition induced by COM, by sustaining the high levels of HK and cellular ATP.
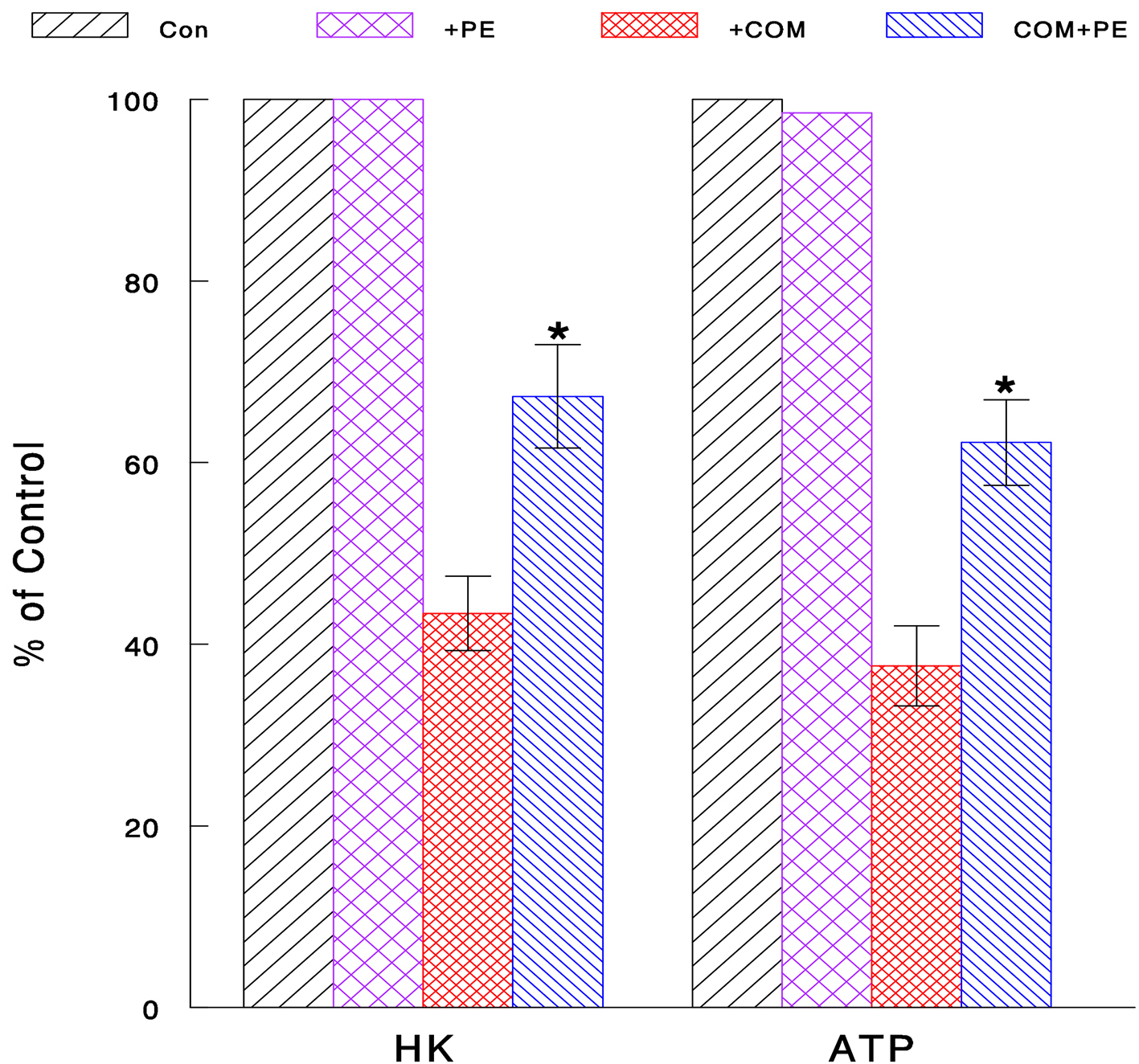 Click for large image | Figure 4. Inhibitory effect of COM on glycolysis and its prevention with PE. Cells exposed to COM (400 μg/mL), PE (50 μg/mL), or PE combined with COM for 6 h were assayed for HK activity and cellular ATP level. All data are mean ± SD from three separate experiments (*P < 0.05 compared with COM-treated cells). |
Effect of COM on cell cycle
As the glycolysis inhibition has been also shown to interfere with cell cycle [25], this possibility was tested next. Cells were exposed to COM (400 μg/mL) or PE (50 μg/mL) alone or their combination (COM + PE) for 6 h and subjected to cell cycle analysis. Compared to controls, the G1-phase cell population increased to 69.2% (i.e., about 41% increase) while the S-phase population decreased to 20.5% (about 47% decrease) with COM exposure (Fig. 5). This accumulation of cells in the G1 phase is known as a G1 cell cycle arrest [26], which would subsequently lead to the growth cessation and cell viability reduction. However, PE significantly decreased the G1 cell population (increased by COM) to 59.1% and increased the S population (reduced by COM) to 30.3% (Fig. 5). These findings suggest that some of cells trapped in the G1 phase were moving/entering to the S phase, partially reversing a G1 cell cycle arrest. Thus, COM may substantially induce a G1 arrest (by interrupting the G1-S cell cycle progression) but PE is capable of partially blocking it, allowing the cells to continue proliferating.
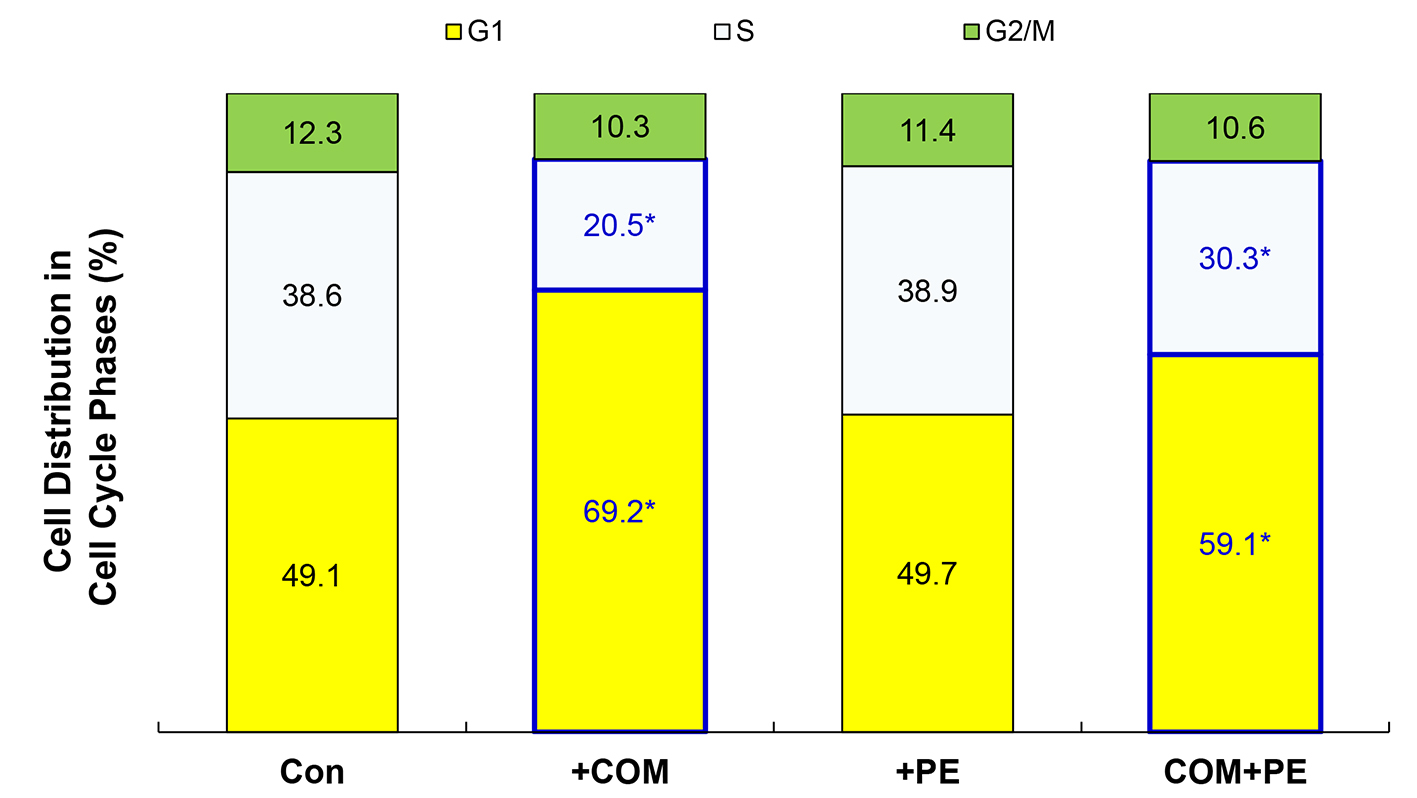 Click for large image | Figure 5. Cell cycle analysis. Cells exposed to COM (400 μg/mL), PE (50 μg/mL), or PE combined with COM for 6 h were subjected to cell cycle analysis. The data are representatives of three independent experiments (*P < 0.05 compared with controls). |
| Discussion | ▴Top |
Despite numerous studies on nephrolithiasis being currently underway, the pathogenesis of kidney stone has not been fully understood. Nowadays, oxidative stress is increasingly believed to play a significant role in nephrolithiasis [8, 9]. CaOx or its crystal form known as COM has been shown to exert detrimental oxidative stress on renal cells, ultimately leading to kidney stone formation [5, 10]. Exploring such a cytotoxic mechanism may give us a better understanding of nephrolithiasis. We also believe that it is crucial to find certain natural agents with the improved preventative effect on stone formation but with few side/adverse effects.
In this study, we examined if a bioactive mushroom extract, PE, might have antioxidant and renoprotective effects on renal tubular epithelial LLC-PK1 cells under oxidative stress mediated through H2O2 or COM. Both H2O2 and COM significantly reduced cell viability with the IC50 of 70 μM and 400 μg/mL for H2O2 and COM, respectively. Such a cell viability reduction was also associated with severe oxidative stress exerted on cells (Fig. 2). However, PE was capable of significantly reducing the severity of such oxidative stress (Fig. 2) but increasing cell viability reduced by oxidative stress (Fig. 3). Therefore, PE may have antioxidant activity against oxidative stress, protecting renal cells from destructive oxidative attack.
We yet know little about how oxidative stress mediated through H2O2 or COM would lead to the cell viability reduction and how PE might prevent it. Nevertheless, it should be noted that our aim was to address if COM would exert oxidative stress, cause renal cell injury, and may eventually facilitate kidney stone formation. We then found that COM did exert oxidative stress (assessed by LPO assay) and induced renal cell injury evidenced by a significant reduction in cell viability. This finding is virtually comparable to what we found in H2O2. It should be reminded again that H2O2 was used as just a positive mediator of oxidative stress in this study.
We next explored how such a COM-induced cell viability reduction was carried out, focusing on glycolysis. It is a metabolic process where glucose goes through the glycolytic pathway and is eventually converted to ATP. This energy (ATP) metabolism is the key to cell proliferation and survival [23, 24]. There are several crucial parameters that can be used to assess the status of glycolysis, and two of such parameters, HK activity and ATP synthesis, were chosen for assessing how glycolysis would be affected by COM-mediated oxidative stress. HK is one of the key glycolytic enzymes involved in the first step in glycolysis, which is also the irreversible committed step [23]. A blocking or inhibition of this step will shut down the rest of the glycolytic pathway, resulting in no new ATP synthesis or critical ATP depletion. In fact, both HK activity and cellular ATP level were significantly reduced by COM (Fig. 4), indicating the inhibition of glycolysis. However, PE significantly increased the COM-reduced HK and ATP levels, implying that the glycolysis inhibition by COM was effectively reversed by PE. Thus, COM-mediated oxidative stress could inhibit glycolysis but PE may prevent such an inhibition to keep cells alive and growing.
In addition, we also examined possible effect of COM-mediated oxidative stress on cell cycle and found a G1 cell cycle arrest having been induced by COM. However, PE partially reversed such a cell cycle blockage, allowing some of cells to move from the G1 to the S phase. Thus, although COM may target cell cycle, PE could yet block it to ensure cell proliferation.
Taken together, the glycolysis inhibition coupled with a cell cycle arrest induced by COM may account for the growth cessation and cell viability reduction in renal (LLC-PK1) cells.
Overall, PE appears to significantly prevent adverse effects mediated through COM, especially protecting renal cells from oxidative attack. This may have significant implication in prevention of CaOx stone formation induced by oxidative stress. During the development of kidney stone, crystallization of CaOx (i.e., COM) is the primary element required for stone formation, but these crystals would never develop into a stone without a “matrix”, such as glycoproteins, glycosaminoglycans, or lipids [27]. For instance, as the brush border membrane of renal epithelial cells is rather susceptible to oxidative stress, its membranous debris (due to cell injury) could likely become such a potential matrix for crystal aggregation [10]. As shown in this study, COM could induce a cell viability reduction through cell injury (directly caused by oxidative stress) and also through the glycolysis inhibition and a cell cycle arrest, which would ultimately result in cell death. It is then plausible that such injured or dead cells could be involved in the COM-cell interaction [9, 10] and/or become a matrix [27], further promoting stone formation. Nevertheless, if oxidative stress (exerted by COM) were effectively diminished or reduced by certain antioxidants such as PE, that could significantly reduce the incidence of kidney stones, owing to prevention of COM formation as well as protection of renal epithelial cells from oxidative attack.
Although the data obtained from this in vitro study look promising and encouraging, more studies using animals (in vivo) are required for confirmation and such study is currently underway in our laboratory.
In conclusion, the present study shows that PE has antioxidant and renoprotective effects against severe oxidative stress exerted by H2O2 or COM. Such oxidative stress would inhibit glycolysis, deplete cellular ATP, and also interfere with the cell cycle progression (a G1 cell cycle arrest). These adverse effects may account for the resulting cell viability reduction but could be effectively prevented by PE. Therefore, PE is a promising agent with antioxidant activity, capable of protecting renal cells from oxidative assault, and may also reduce the incidence of CaOx stone cases. Further studies are warranted.
Acknowledgments
We thank Mike Shirota and Donna Noonan (Mushroom Wisdom, Inc.) for generously providing us with Poria mushroom extract and their courteous assistance in this study.
Financial Disclosure
No competing financial interests exist.
| References | ▴Top |
- Baud L, Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986;251(5 Pt 2):F765-776.
pubmed - Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997;29(3):465-477.
doi - Diamond JR. The role of reactive oxygen species in animal models of glomerular disease. Am J Kidney Dis. 1992;19(3):292-300.
doi - Andreoli SP, McAteer JA. Reactive oxygen molecule-mediated injury in endothelial and renal tubular epithelial cells in vitro. Kidney Int. 1990;38(5):785-794.
doi - Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157(3):1059-1063.
doi - Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, et al. Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int. 1996;49(2):413-419.
doi pubmed - Hesse A, Siener R. Current aspects of epidemiology and nutrition in urinary stone disease. World J Urol. 1997;15(3):165-171.
doi pubmed - Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS. Calcium oxalate crystal attachment to cultured kidney epithelial cell lines. J Urol. 1998;160(4):1528-1532.
doi - Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int. 2001;59(2):637-644.
doi pubmed - Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31(1):3-9.
pubmed - Selvam R. Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res. 2002;30(1):35-47.
doi pubmed - Fishman AI, Green D, Lynch A, Choudhury M, Eshghi M, Konno S. Preventive effect of specific antioxidant on oxidative renal cell injury associated with renal crystal formation. Urology. 2013;82(2):489 e481-487.
- Rios JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011;77(7):681-691.
doi pubmed - Wang Y, Zhang M, Ruan D, Shashkov AS, Kilcoyne M, Savage AV, Zhang L. Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos. Carbohydr Res. 2004;339(2):327-334.
doi pubmed - Zhao YY, Lei P, Chen DQ, Feng YL, Bai X. Renal metabolic profiling of early renal injury and renoprotective effects of Poria cocos epidermis using UPLC Q-TOF/HSMS/MSE. J Pharm Biomed Anal. 2013;81-82:202-209.
doi pubmed - Chen X, Zhang L, Cheung PC. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1-->3)-d-glucan from Poria cocos. Int Immunopharmacol. 2010;10(4):398-405.
doi pubmed - Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, Smith PT, et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med. 2013;6(9):673-681.
doi - Zhou L, Zhang Y, Gapter LA, Ling H, Agarwal R, Ng KY. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem Pharm Bull (Tokyo). 2008;56(10):1459-1462.
doi - Li TH, Hou CC, Chang CL, Yang WC. Anti-Hyperglycemic Properties of Crude Extract and Triterpenes from Poria cocos. Evid Based Complement Alternat Med. 2011;2011.
- Fuchs SM, Heinemann C, Schliemann-Willers S, Hartl H, Fluhr JW, Elsner P. Assessment of anti-inflammatory activity of Poria cocos in sodium lauryl sulphate-induced irritant contact dermatitis. Skin Res Technol. 2006;12(4):223-227.
doi pubmed - Feng YL, Lei P, Tian T, Yin L, Chen DQ, Chen H, Mei Q, et al. Diuretic activity of some fractions of the epidermis of Poria cocos. J Ethnopharmacol. 2013;150(3):1114-1118.
doi pubmed - Dargel R. Lipid peroxidation - a common pathogenetic mechanism? Exp Toxicol Pathol. 1992;44(4):169-181.
doi - Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633-4646.
doi pubmed - Simons AL, Mattson DM, Dornfeld K, Spitz DR. Glucose deprivation-induced metabolic oxidative stress and cancer therapy. J Cancer Res Ther. 2009;5(Suppl 1):S2-6.
doi pubmed - Loar P, Wahl H, Kshirsagar M, Gossner G, Griffith K, Liu JR. Inhibition of glycolysis enhances cisplatin-induced apoptosis in ovarian cancer cells. Am J Obstet Gynecol. 2010;202(4):371 e371-378.
- Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60(14):3689-3695.
pubmed - Khan SR, Glenton PA, Backov R, Talham DR. Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int. 2002;62(6):2062-2072.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.











