| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 15, Number 10-11, December 2023, pages 446-455
Upadacitinib Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis for Ulcerative Colitis Achieved Ulcer Healing for Pyoderma Gangrenosum
Satoshi Tanidaa, b, d, Ryoji Kuboc, Shoichiro Yoshiic, Takuya Takahamab, Shun Sasohb, Yoshimasa Kubotab, Tesshin Banb, Tomoaki Andob, Makoto Nakamurab, Takashi Johb
aEducation and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
bDivision of Gastroenterology, Gamagori City Hospital, Hirata, Gamagori, Aichi 443-8501, Japan
cDivision of Dermatology, Gamagori City Hospital, Hirata, Gamagori, Aichi 443-8501, Japan
dCorresponding Author: Satoshi Tanida, Education and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
Manuscript submitted August 2, 2023, accepted October 13, 2023, published online November 3, 2023
Short title: Upadacitinib Plus Intensive GMA for PG and UC
doi: https://doi.org/10.14740/jocmr5005
| Abstract | ▴Top |
A 44-year-old woman who had been diagnosed with ulcerative colitis (UC) at 22 years old was diagnosed with severe flare-up of UC based on endoscopic findings associated with new-onset active pyoderma gangrenosum (PG) on both lower legs after she decided to discontinue UC treatment. Systemic treatment with intravenous prednisolone at 30 mg/day had achieved insufficient response to UC and PG, resulting in a diagnosis of corticosteroid-refractory UC and PG. Combination therapy with upadacitinib at 45 mg/day plus intensive granulocyte and monocyte adsorptive apheresis (GMA) was started to achieve clinical remission of UC. Ten weeks after starting this combination therapy, clinical improvement of UC was achieved with PG ulcer healing on both lower legs. A combination of upadacitinib plus intensive GMA may offer an effective therapeutic option for patients with active PG in addition to UC but has yet to be approved for induction or maintenance treatment of PG worldwide. PG is a dermatological involvement in UC patients that requires attention.
Keywords: Ulcerative colitis; New-onset pyoderma gangrenosum; Upadacitinib; Intensive granulocyte and monocyte adsorptive apheresis; Ulcer healing
| Introduction | ▴Top |
Pyoderma gangrenosum (PG) is a rare dermatological disorder characterized by a rapidly progressing necrotic ulcer with erythematous lesions in the skin [1]. This disease shows a sex predilection for female individuals [2]. The etiology of PG remains unclear but has been attributed to reactive neutrophilic dermatosis [3]. The histopathological findings of PG are nonspecific but show intense neutrophilic infiltrates, neutrophilic pustules, and abscess with surrounding mononuclear cell infiltrates in severe skin lesions [4]. The pathogenesis of PG is proposed to involve neutrophil-mediated autoinflammatory disease via increased production of cytokines, chemotactic factors, and matrix metalloproteinases (MMPs) induced by neutrophils and T lymphocytes [5]. Another mechanism underlying the deterioration of PG lesions is upregulation of the Janus kinase (JAK) pathway [6]. Current pharmacotherapies for PG include topical corticosteroid therapy [7] with concomitant use of systemic corticosteroids [8] and oral cyclosporin [9]. Biologics including anti-tumor necrosis factor (TNF)-α antibodies can also be selected for patients showing inadequate response to conventional therapies and active moderate or severe disease [10]. Adalimumab, a monoclonal antibody that binds to TNF-α and thus blocks the TNF-α signaling pathway, was first approved in 2020 in Japan [10]. However, JAK inhibitors have not been approved for the treatment of active PG. PG is often associated with immune-mediated diseases such as inflammatory bowel diseases (IBD) (ulcerative colitis (UC) and Crohn’s disease), rheumatoid arthritis and hematological conditions [11-13].
UC is characterized by mucosal ulceration, rectal bleeding, diarrhea, and abdominal pain. Treatments for moderate-to-severe UC include 5-aminosalicylic acids, corticosteroids, thiopurines, biologics (neutralizing antibodies against TNF-α [14, 15], interleukin (IL)-12 and/or IL-23 [16] and integrins [17]), a small molecule antagonist targeting integrin-α4 [18] and JAK inhibitors [19, 20]. Upadacitinib, a JAK inhibitor which is selective for JAK1, was approved for the treatment of moderate-to-severe UC in Japan in 2022 [21].
Intensive granulocyte and monocyte adsorptive apheresis (GMA) is also available in Europe and Japan for the treatment of active UC that may or may not be refractory to standard pharmacotherapies, biologics and JAK inhibitors [22, 23].
We report herein a rare case in which combination induction therapy with upadacitinib plus intensive GMA for the treatment of PG and UC rapidly achieved clinical improvement and histologic mucosal improvement of UC and healing of PG ulcers within 10 weeks in a patient with new-onset PG associated with acute severe UC deterioration, who had been refractory to treatment with systemic corticosteroids.
| Case Report | ▴Top |
Investigations
This patient was diagnosed with left-sided type UC in the beginning of 2001. Oral salazosulfapyridine was started at 2,000 mg/day and subsequent treatment with weekly GMA (total 10 sessions) immediately induced clinical remission. She had independently made the decision to discontinue salazosulfapyridine treatment in 2002 but had spontaneously remained in clinical remission. The patient was subsequently diagnosed with erythema nodosum on both lower legs in August 2022, at 43 years old. The diagnosis was based on pathological findings of septal panniculitis showing massive infiltration of inflammatory cells comprising mononuclear cells and/or neutrophils, some Langhans giant cell aggregations, focal fibrosis, and no vasculitis in the subcutaneous adipose tissue. Oral loxoprofen sodium hydrate was started at 180 mg/day along with topical corticosteroid ointment. According to the condition of skin eruptions, oral loxoprofen sodium hydrate and topical corticosteroid ointment had been intermittently administered for 2 years. Erythema nodosum was finally resolved. In March 2023, at 44 years old, deep ulcers with erythema appeared on the flexor surfaces of both lower legs (Fig. 1a). Pathological findings of biopsy specimens from deep ulcers on both lower legs showed necrotizing suppurative inflammation and perivascular infiltrates due to dominant neutrophils rather than lymphocytes with lack of vasculitis in the dermis to subcutaneous adipose tissue, and focal fibrosis in the dermis, consistent with PG (Fig. 1b, c). As UC flared up concomitantly with repeated episodes of diarrhea and bloody stool (≥ 10 times/day), she was referred to our division by the Division of Dermatology. The patient had no history of lung or joint disease and deep venous thrombosis, and no family history of UC or PG. The patient’s height and weight were 159 cm and 58.6 kg, respectively. The body mass index was 23.1. Body weight loss (-5 kg) was observed. Laboratory investigations showed: white blood cell (WBC) count: 11,300/µL; red blood cell (RBC) count: 372 × 104/µL; hemoglobin (Hb): 10.6 g/dL; total protein (TP): 6.6 g/dL; albumin (Alb): 3.0 g/dL; aspartate aminotransferase (AST): 14 U/L; alanine aminotransferase (ALT): 14 U/L; Fe: 13 µg/dL; and C-reactive protein: 5.54 mg/dL. Proteinase (PR)3-antineutrophil cytoplasmic antibody (ANCA) and myeloperoxidase (MPO)-ANCA were 2.3 and < 1.0, respectively. A negative result was obtained from an antigenemia (C-7HRP) test for cytomegalovirus. Further, neither the pathogenic microbe Clostridium difficile nor its A or B toxins were detected from stool cultures (Table 1). Colonoscopy on admission in March 2023 showed marked erythema and ulcerations with spontaneous bleeding from the transverse colon to the rectum (Mayo endoscopic subscore, 3) (Fig. 2a). Histopathological findings of biopsy specimens taken from the area of severest inflammation based on Geboes score revealed a marked increase of neutrophils in the lamina propria with unequivocal crypt destruction and ulceration due to neutrophil infiltration (grade 5.4) (Fig. 2b) [24]. Immunohistochemical examination of colon biopsy specimens for cytomegalovirus also yielded negative results. Pain and discomfort from PG ulcers were evaluated using a visual analogue scale (VAS).
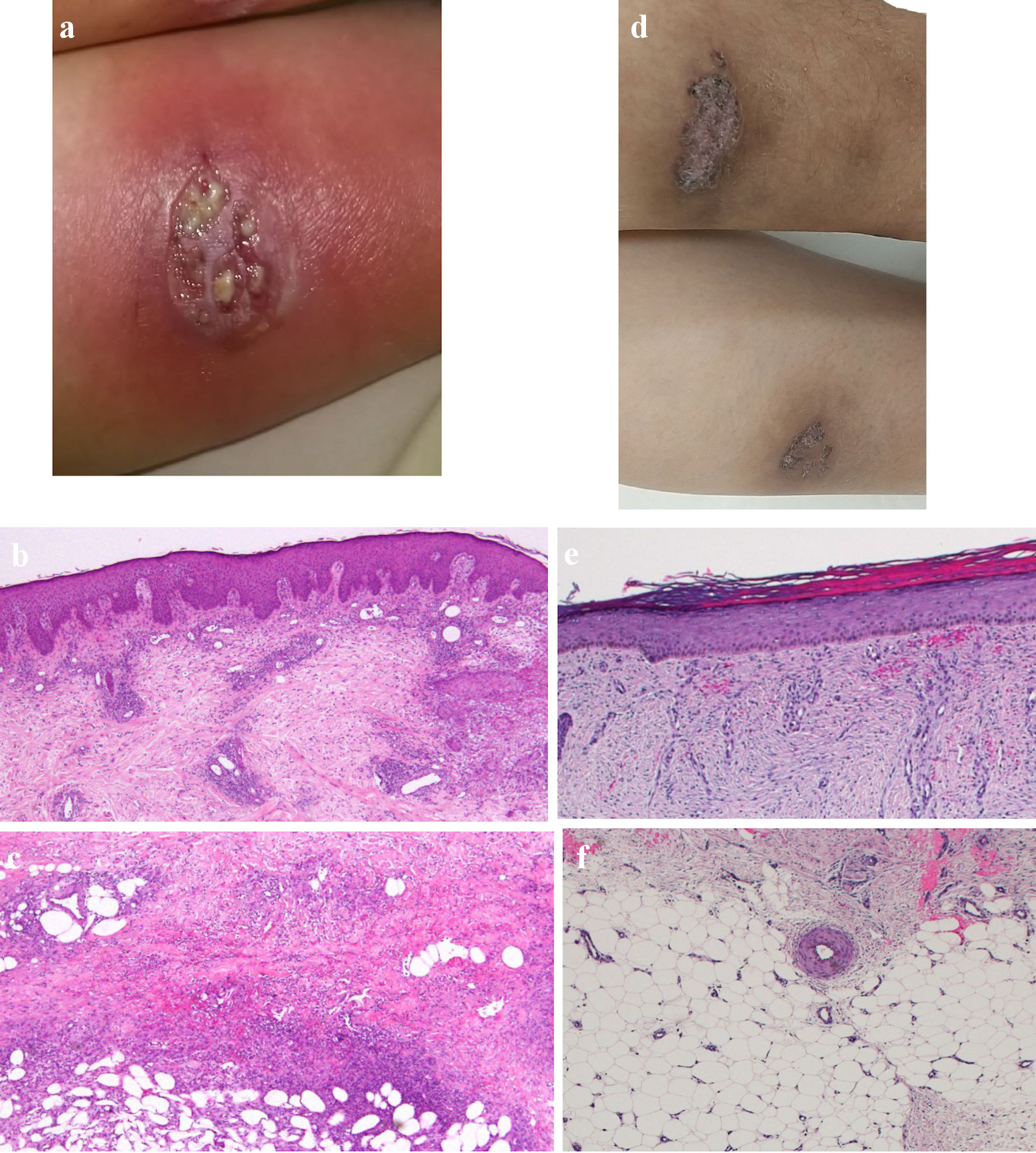 Click for large image | Figure 1. Skin findings of pyoderma gangrenosum on the lower legs. (a) On admission, gross inspection shows deep ulcers with erythema on the flexion side of the left lower leg as the representative lesion. (b, c) On admission, histopathological findings show diffusely necrotizing suppurative inflammation and perivascular infiltrates with lack of vasculitis due to dominant neutrophils rather than lymphocytes, and focal fibrosis in the dermis (b, × 40), and necrotizing suppurative inflammation in the subcutaneous adipose tissue due to dominant neutrophils (c, × 40). (d) At 10 weeks after starting combination therapy, gross inspection shows healing of the ulcer with re-epithelization. (e, f) At 10 weeks after starting combination therapy, ulcers show re-epithelization with mild edema and regeneration of venules in the dermis (e, × 40), and mild fibrosis in the subcutaneous tissue (f, × 40). |
 Click to view | Table 1. Laboratory Findings on Admission |
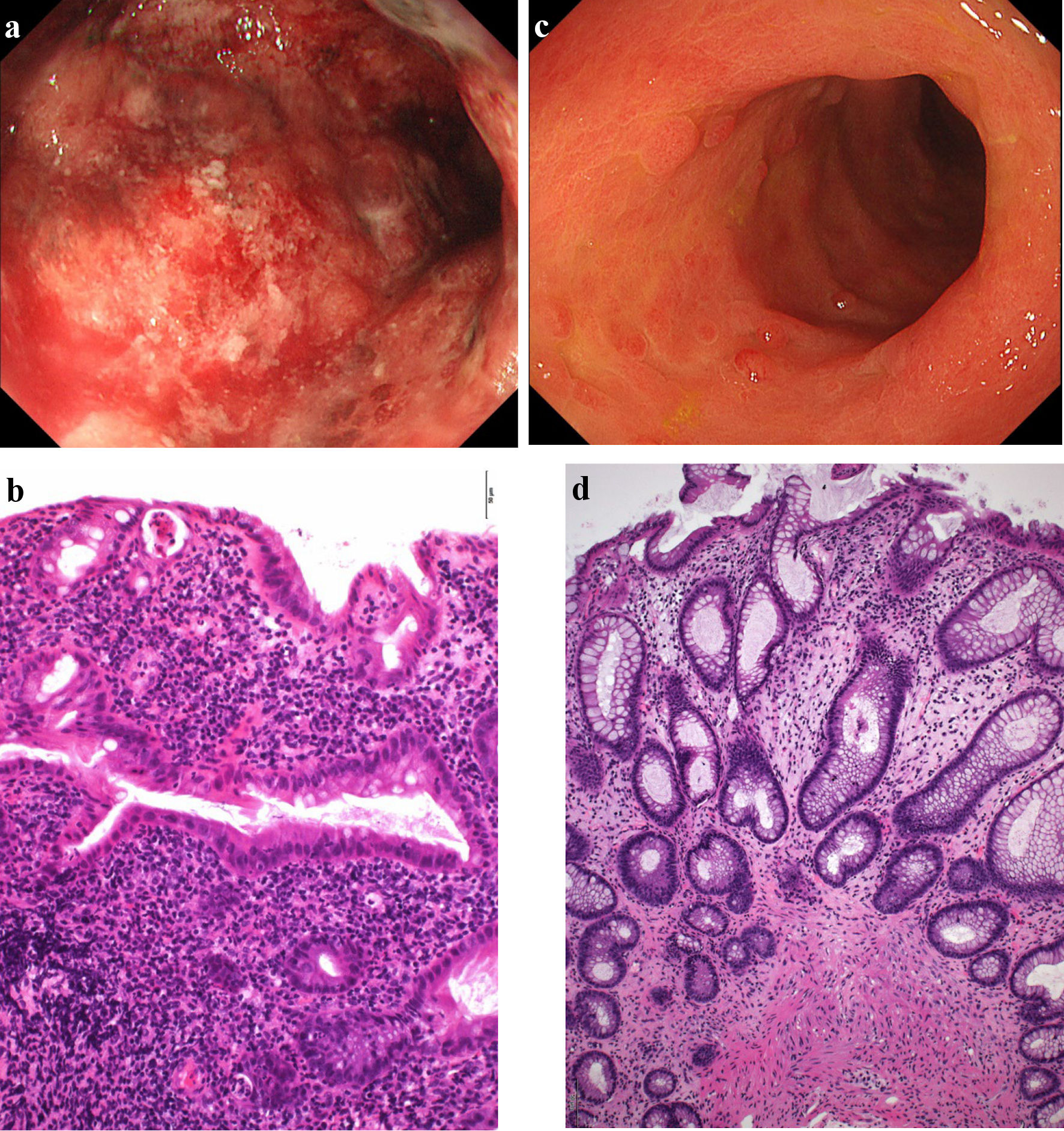 Click for large image | Figure 2. Findings from colonoscopy. (a) On admission, colonoscopy of the descending colon shows edematous mucosa and ulcerations with marked erythema and spontaneous bleeding. (b) On admission, histopathological findings based on Geboes score (grade 5.4) show severe diffuse or multifocal abnormalities of crypts and a marked increase in chronic inflammatory infiltrates, with no increase in eosinophils and a marked increase in neutrophils in the lamina propria. Over 50% of crypts in the epithelium showed infiltration of neutrophils, unequivocal crypt destruction and ulcer or granulation tissue (× 200). (c) At 10 weeks after starting combination therapy, colonoscopy of the descending colon shows marked erythema, no clear vascular pattern with no ulcerated surfaces. (d) At 10 weeks after starting combination therapy, histopathological findings based on Geboes score (grade 2A.1) show no crypt abnormality, a mild increase in chronic inflammatory infiltrates, a mild increase of eosinophils and no increase of neutrophils in the lamina propria, and no neutrophil infiltration in the epithelial crypts, no crypt destruction and no erosion, ulceration, or granulation tissue (× 100). |
Diagnosis
Based on all these findings, the patient was diagnosed with severe UC flare (full Mayo score: 12; stool frequency score: 3; rectal bleeding: 3; mucosal appearance at endoscopy: 3; physician global assessment: 3) and new-onset active PG.
Treatment, follow-up and outcomes
The patient was admitted to our hospital due to rapid exacerbation of additional symptoms with fever ≥ 38.0 °C and severe pain from PG lesions. Prednisolone (PSL) was intravenously administered at 30 mg/day along with 4,800 mg/day of mesalazine in the middle of March 2023. Total parenteral nutrition was administered because of repeated diarrhea and bloody stools till the beginning of April. In addition, a total of 300 mL (1 kcal/mL) of supplementary enteral nutrition in a day with Elental® (EA Pharma Co., Ltd., Tokyo, Japan) was perorally started because one randomized trial comparing the efficacy of total enteral nutrition to total parenteral nutrition in patients with acute severe UC during intensive corticosteroid therapy showed that an increased level of serum albumin in the enteral nutrition group was significantly greater than that in the total parenteral nutrition group [25]. Moreover, enteral nutrition has been shown to have an anti-inflammatory effect [26] and to enhance wound healing in the intestinal mucosa [27]. However, the patient could not continue supplementary enteral nutrition because of its unfavorite taste. Antibiotics (cefazolin) were intravenously provided at 2 g/day for a week to prevent suppurative infection after biopsy of PG ulcers and from bacterial adhesion to ulcerated surfaces. However, 7 days after starting PSL, bloody diarrhea and the appearance of PG ulcer showed little improvement and combination therapy with 45 mg/day of upadacitinib plus intensive GMA (two sessions a week; total, 10 times) was started for corticosteroid-refractory UC. Ten days after starting oral mesalazine, watery diarrhea was exacerbated and was accompanied with spike fevers and abnormally elevated liver transaminases and biliary tract enzymes (AST: 392 U/L; ALT: 437 U/L; alkaline phosphatase (ALP): 206 U/L; γ-glutamyl transferase (GTP): 281 U/L). Hepatitis B surface (HBs) antigen and antibody, hepatitis B core (HBc) antibody, hepatitis B virus (HBV) DNA and hepatitis C virus (HCV) antibody were all negative. Epstein-Barr virus (EBV) capsid antigen immunoglobulin (Ig) G, IgM and EBV nuclear antigen (EBNA) values were 80 times, < 10 times and 40 times, respectively, compatible with past infection. Anti-nuclear antibodies were also negative. These findings suggested that the patient was allergic to mesalazine. Watery diarrhea and spike fever disappeared, and liver transaminases and biliary tract enzymes normalized immediately after discontinuing mesalazine. As of the end of March 2023, PG ulcers had re-epithelized, resulting in ulcer healing and improvement of symptoms including diarrhea, bloody stools and abdominal pain attributed to UC. PSL was subsequently tapered to 10 mg/day after 18 days of administration. In the beginning of April, 10 days after starting upadacitinib, mild anemia was detected (RBC: 328 × 104/µL; Hb: 9.9 mg/dL). Because upadacitinib was considered to cause anemia, the dose of upadacitinib was decreased to 30 mg/day. RBC count normalized, and the patient was discharged.
The dose of PSL was tapered off by the end of April. At the beginning of June, 10 weeks after starting combination therapy with upadacitinib plus intensive GMA, PG on the lower legs showed ulcer healing with re-epithelization (Fig. 1d). Pathological findings for biopsy specimens from PG scars showed ulcer re-epithelization with mild edema and regeneration of venules in the dermis, and mild fibrosis in the subcutaneous tissue (Fig. 1e, f). Follow-up endoscopy showed marked erythema, no clear vascular pattern with no ulcerated surfaces (Mayo endoscopic mucosal appearance: 2) (Fig. 2c), resulting in a full Mayo score of 3 (stool frequency score: 0; rectal bleeding: 0; physician global assessment: 1). Histopathological findings of colon biopsies taken from the area of severest inflammation based on Geboes score revealed a mild increase in eosinophils and no increase in neutrophils in the lamina propria, and no erosion and ulceration (grade 2A.1), reflecting histologic improvement of the mucosa (Fig. 2d). VAS for PG ulcer pain decreased from full score to 0 as the surface area of ulcered skin reduced. As of 3 months after admission, the patient remained in a state of stable PG ulcer healing and UC improvement under maintenance treatment with upadacitinib at 30 mg/day (Fig. 3). No symptoms and manifestations including pains, discolored skin, venous ectasia, and varices at extremities based on deep venous thromboses were observed after upadacitinib administration. A contrast-enhanced whole-body computed tomography (CT) from head to extremities after upadacitinib administration did not show any deep venous thromboses. Muscle pains and elevated values of creatine phosphokinase were also not observed after upadacitinib administration.
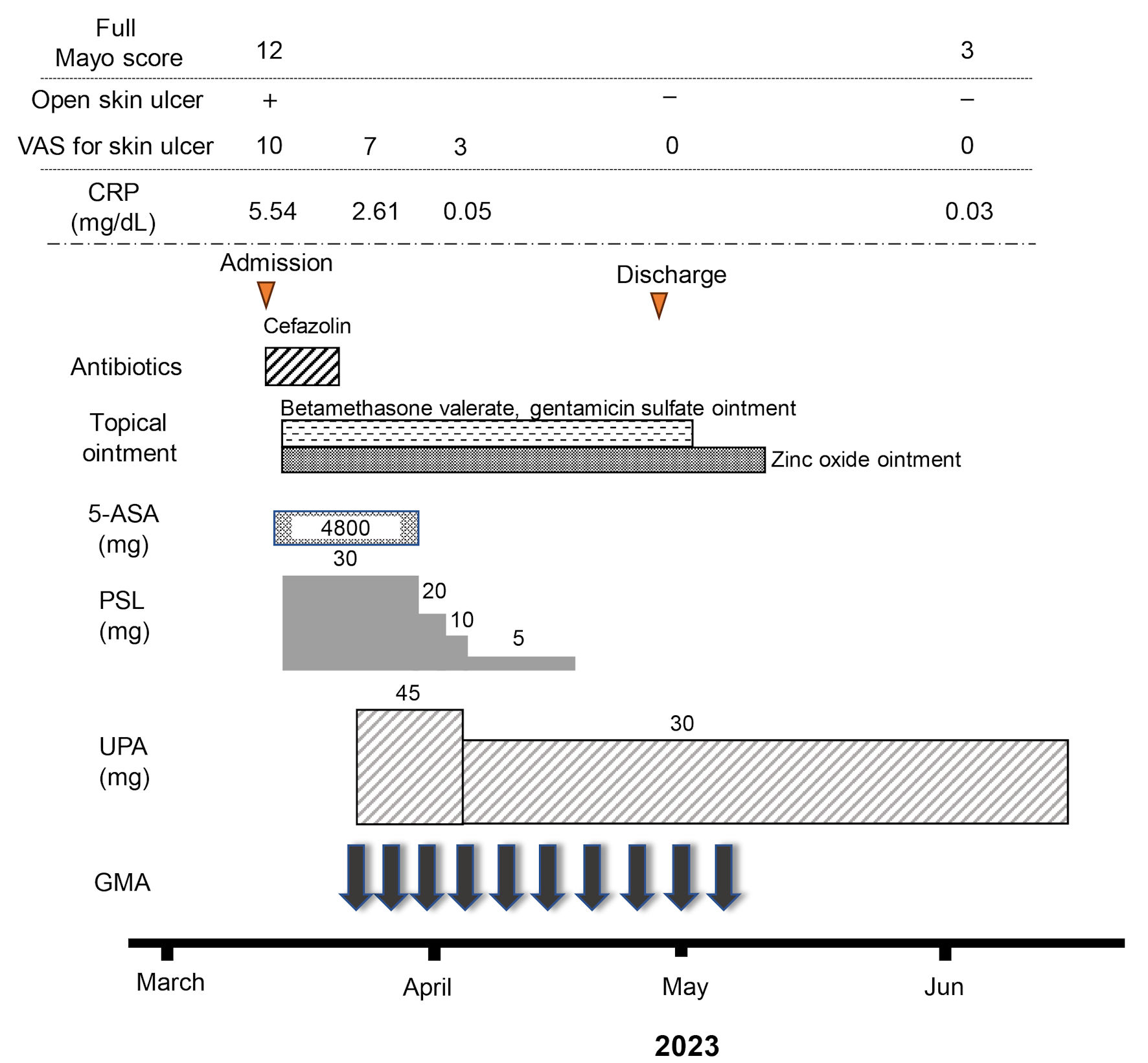 Click for large image | Figure 3. Clinical course for 10 weeks. The patient was admitted for treatment of active pyoderma gangrenosum (PG) associated with severe ulcerative colitis (UC) in March, 2023. Full Mayo score on admission as an activity index for UC was 12, including a Mayo endoscopic subscore of 3. Intravenous administration of prednisolone (PSL) was started at 30 mg/day, accompanied by mesalazine at 4,800 mg/day. However, combination therapy with upadacitinib (UPA) at 45 mg/day plus intensive granulocyte and monocyte adsorptive apheresis (GMA) was started as corticosteroid-refractory disease was seen. Mesalazine was then discontinued because the patient proved allergic to the drug. In addition, the dose of upadacitinib was decreased from 45 to 30 mg/day because anemia was identified 10 days after starting upadacitinib. An ointment combining betamethasone valerate and gentamicin sulfate and zinc oxide ointment had been continued until the PG ulcer healed. The dose of PSL was tapered off at the end of April. At the beginning of June, after 10 weeks of combination therapy using upadacitinib plus intensive GMA, clinical improvement (full Mayo score, 3), histologic mucosal improvement of UC, ulcer healing and marked histologic improvement of PG were achieved. 5-ASA: 5-aminosalicylic acid; CRP: C-reactive protein. |
| Discussion | ▴Top |
We have reported herein a rare case in which upadacitinib plus intensive GMA for UC achieved clinical improvement and histologic mucosal improvement of UC and ulcer healing and marked histologic improvement of new-onset PG on the lower legs within 10 weeks.
PG is a rare, intractable, neutrophilic dermatosis often associated with immune-mediated diseases such as IBD and rheumatoid arthritis. The prevalence of PG as a comorbidity among patients with UC in Japan is 23.5% [28]. As a comorbidity of PG, UC is the most frequently prevalent of the immune-mediated diseases, followed by rheumatoid arthritis (9.7%) and Crohn’s disease (7.2%). On the other hand, a systematic review and meta-analysis based on data reported from 379 cases of PG in a cumulative cohort of 61,695 IBD patients (including Crohn’s disease and UC) showed that Crohn’s disease was associated with an increased pooled relative risk of PG (risk ratio: 1.193; 95% confidence interval: 1.001 - 1.422; P = 0.048), but, no association between UC and relative risk of PG was observed [29]. Thus, new-onset PG in patients with UC appears rare. Moreover, a case-control study investigating serologic factors associated with PG in IBD (UC and Crohn’s disease) patients has shown that Crohn’s disease patients, particularly colitis-type, associated with PG are more likely to be positive for perinuclear ANCA, but UC patients with PG does not correlate with perinuclear ANCA [30]. In the present case, both serologic values of MPO-ANCA as perinuclear ANCA and PR3-ANCA as cytoplasmic ANCA were low; and pathological findings from active PG ulcer showed lack of vasculitis. Based on these outcomes, this new-onset PG patient with UC was not likely to correlate with ANCA-associated vasculitis.
Regarding genetic factors associated with disease development of PG in IBD patients, a genome-wide association genotyping and multivariable logistic regression analysis has shown that some known IBD susceptibility gene loci comprising IL-8α and tissue inhibitor of matrix metalloproteinase 3 (TIMP-3) are significantly associated with PG, [30]. However, these genes cannot be examined in the clinical setting in Japan.
In terms of pathogenesis, neutrophils are known to play a key role in the progression of PG. Upregulation of many proinflammatory and neutrophil chemotactic factors (TNF-α, IL-8, IL-1β, and MMP-2, -9 and -10), and CC chemokine receptor (CCR)-5 in skin lesions is induced both by upregulation of aberrant inflammasomes in the innate immune system and by type 1 helper T (Th1) cells and type 17 helper T (Th17) cells in the adaptive immune system [31, 32]. IL-8 has been demonstrated to produce PG in animal models [33]. MMP-9 and -10 contribute to poor healing of PG [34]. Based on the molecular pathogenesis background, treatment for PG has relied on anti-TNF-α antibodies in addition to traditional immunosuppressants. PG and UC are immune-mediated refractory diseases presenting with a relapsing-remitting clinical course. As relapses and resistances for several different classes of drugs are frequent, no standardized therapy has yet been determined. PG does not appear to always respond to immunosuppressive medications conventionally used for IBD [35]. Thus, PG therapy is often challenging when associated with other immune-mediated diseases such as UC. GMA with Adacolumn® (JIMRO, Takasaki, Japan) is an efficacious and safe therapeutic option for patients with mild-to-moderate UC that proves refractory to pharmacotherapy [36]. A case series investigating the efficacy of weekly GMA (one session a week; total, 10 or 11 times) in three refractory PG patients showed re-epithelialization of ulcers in two patients and a reduced lesion size in the remaining one. Therefore, GMA has been proposed as an effective alternative to current conventional pharmacotherapy with regard to refractory cases of PG [37]. Moreover, recent studies have shown a possible new target for PG therapy based on the apparent efficacy of JAK inhibitors for refractory PG [38, 39]. Upadacitinib enhanced selective inhibition of JAK1 has the potential to improve the benefit-to-risk profile in patients with inflammatory diseases by minimizing the inhibitory effects on other JAK isoforms, compared to less selective JAK inhibitors [40, 41]. In addition, in two multicenter, double-blinded placebo-controlled trials of patients with moderate-to-severe active UC, at week 8, upadacitinib could achieve histologic-endoscopic mucosal improvement (30% and 36% vs. placebo 6% and 6%, respectively, P < 0.001) (Mayo endoscopic subscore ≤ 1; Geboes score ≤ 3.1) and mucosal healing (10.7% and 13.5% vs. placebo 1.3% and 1.7%, respectively, P < 0.001) (Mayo endoscopic subscore = 0; Geboes score < 2.0) [42]. In the present case, colonoscopy showed severe disease (Mayo endoscopic subscore: 3) with severe histopathological inflammations. The use of upadacitinib monotherapy has been limited when higher induction remission rates are the goal. In addition, combination therapy with JAK inhibitor plus intensive GMA was well tolerated and may be useful for inducing clinical remission and mucosal healing in patients with refractory UC [43]. Such evidence suggests that the addition of intensive GMA to upadacitinib may be more effective than upadacitinib monotherapy because GMA depletes elevated and activated myeloid lineage leucocytes and has been associated with a marked downregulation of inflammatory cytokines, including the IL-1β, IL-6, IL-8, and TNF-α released by myeloid leucocytes and lymphocytes [44]. Thus, upadacitinib plus intensive GMA for the treatment of active UC may markedly reduce inflammatory cell infiltration in both the skin and colon mucosa, resulting in not only ulcer healing and histologic remission of new-onset PG, but also clinical and histologic mucosal remission of UC. In the present case, this combination therapy rapidly achieved clinical improvement and histologic improvement of UC, and ulcer healing and marked histologic improvement of PG within 10 weeks. Interestingly, in the present case, the development of PG ulcers coincided with the exacerbation of UC, similar to a previous study [35]. The optimal dose of upadacitinib for treating PG is unknown, as clinical trials for the efficacy and safety of upadacitinib in patients with active PG remain lacking. According to a phase 3 trial of patients with atopic dermatitis as an immune-mediated dermatological disease, upadacitinib can be increased up to 30 mg/day if the standard dose of 15 mg/day proves inadequate [45]. On the other hand, doses of JAK inhibitors for the treatment of PG in UC patients were determined based on the daily optimal and maximum induction doses for the treatment of UC [39]. In the present case, upadacitinib was provided at 45 mg/day as the optimal induction dose for the treatment of UC [21, 46], in combination with intensive GMA. Clinical improvement and histologic improvement of the mucosa in UC and ulcer healing and marked histologic improvement of PG on the lower legs were finally achieved within 10 weeks. To the best of our knowledge, no previous reports have described upadacitinib plus intensive GMA achieving clinical improvement and histologic mucosal improvement for UC and ulcer healing and marked histologic improvement of PG in a patient with new-onset PG associated with acute severe UC deterioration, who had been refractory to treatment with systemic corticosteroids. Further prospective investigation is needed to determine the doses of upadacitinib adequate to achieve ulcer healing and histologic remission of PG.
Learning points
Combination therapy comprising upadacitinib plus intensive GMA appears effective as a therapeutic option for patients with PG and UC, although approval of upadacitinib for the treatment of PG has not yet been given in Japan. PG is a rare dermatological involvement that requires attention in UC patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Satoshi Tanida has received research grants from AbbVie. None of the other authors has any conflict of interest to declare.
Informed Consent
Informed consent was obtained in the form of opt-out on the website during enrollment.
Author Contributions
ST, SY and RK assisted with and supported sample collection and drafted the manuscript. TT, SS, YK, TB, and TA performed critical editing of the manuscript. ST prepared and wrote the manuscript. MN and TJ carefully supervised this case.
Data Availability
The data supporting the findings of this case are available from the corresponding author upon reasonable request.
Abbreviations
PG: pyoderma gangrenosum; MMPs: matrix metalloproteinases; JAK: Janus kinase; TNF: anti-tumor necrosis factor; IBD: inflammatory bowel disease; UC: ulcerative colitis; IL: interleukin; GMA: granulocyte and monocyte adsorptive apheresis; WBC: white blood cell; RBC: red blood cell; Hb: hemoglobin; TP: total protein; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PR: proteinase; ANCA: antineutrophil cytoplasmic antibody; MPO: myeloperoxidase; VAS: visual analogue scale; PSL: prednisolone; ALP: alkaline phosphatase; GTP: glutamyltransferase; HBs: hepatitis B surface; HBc: hepatitis B core; HBV: hepatitis B virus; HCV: hepatitis C virus; EBV: Epstein-Barr virus; Ig: immunoglobulin; EBNA: Epstein Barr virus nuclear antigen; Th1: type 1 helper T cell; Th17: type 17 helper T cell
| References | ▴Top |
- Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132(9):2166-2170.
doi pubmed - Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB, Jr., White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore). 2000;79(1):37-46.
doi pubmed - Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9(1):39.
- Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790-800.
doi pubmed - Braun-Falco M, Kovnerystyy O, Lohse P, Ruzicka T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66(3):409-415.
doi pubmed - Ortega-Loayza AG, Nugent WH, Lucero OM, Washington SL, Nunley JR, Walsh SW. Dysregulation of inflammatory gene expression in lesional and nonlesional skin of patients with pyoderma gangrenosum. Br J Dermatol. 2018;178(1):e35-e36.
doi pubmed - Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges. 2015;13(4):317-324.
doi pubmed - Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14(3):225-233.
doi pubmed - Mason JM, Thomas KS, Ormerod AD, Craig FE, Mitchell E, Norrie J, Williams HC, et al. Ciclosporin compared with prednisolone therapy for patients with pyoderma gangrenosum: cost-effectiveness analysis of the STOP GAP trial. Br J Dermatol. 2017;177(6):1527-1536.
doi pubmed pmc - Yamasaki K, Yamanaka K, Zhao Y, Iwano S, Takei K, Suzuki K, Yamamoto T. Adalimumab in Japanese patients with active ulcers of pyoderma gangrenosum: Twenty-six-week phase 3 open-label study. J Dermatol. 2020;47(12):1383-1390.
doi pubmed pmc - Salmon Olavarria P, Rubio Iturria S, Nantes Castillejo O. Tofacitinib, a useful option for the treatment of pyoderma gangrenosum in an ulcerative colitis patient. Rev Esp Enferm Dig. 2021;113(10):733-734.
doi pubmed - Kochar B, Herfarth N, Mamie C, Navarini AA, Scharl M, Herfarth HH. Tofacitinib for the treatment of pyoderma gangrenosum. Clin Gastroenterol Hepatol. 2019;17(5):991-993.
doi pubmed - Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, Winthrop KL, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76(7):1253-1262.
doi pubmed pmc - Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462-2476.
doi pubmed - Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, Kron M, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257-265.e251-253.
doi pubmed - Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201-1214.
doi pubmed - Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699-710.
doi pubmed - Matsuoka K, Watanabe M, Ohmori T, Nakajima K, Ishida T, Ishiguro Y, Kanke K, et al. AJM300 (carotegrast methyl), an oral antagonist of alpha4-integrin, as induction therapy for patients with moderately active ulcerative colitis: a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol Hepatol. 2022;7(7):648-657.
doi pubmed - Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723-1736.
doi pubmed - Feagan BG, Danese S, Loftus EV, Jr., Vermeire S, Schreiber S, Ritter T, Fogel R, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372-2384.
doi pubmed - Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hebuterne X, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128.
doi pubmed - Tanida S, Mizoshita T, Nishie H, Ozeki K, Katano T, Kubota E, Kataoka H, et al. Combination Therapy With Adalimumab Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis in Patients With Refractory Ulcerative Colitis. J Clin Med Res. 2015;7(11):884-889.
doi pubmed pmc - Tanida S, Ozeki K, Katano T, Tanaka M, Shimura T, Kubota E, Kataoka H, et al. Induction Therapy With a Combination of Weekly Adalimumab Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis in Patients With Ulcerative Colitis and Failure of Conventional Agents, Biologics and Janus Kinase Inhibitor. J Clin Med Res. 2023;15(3):181-186.
doi pubmed pmc - Bessissow T, Lemmens B, Ferrante M, Bisschops R, Van Steen K, Geboes K, Van Assche G, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107(11):1684-1692.
doi pubmed - Gonzalez-Huix F, Fernandez-Banares F, Esteve-Comas M, Abad-Lacruz A, Cabre E, Acero D, Figa M, et al. Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am J Gastroenterol. 1993;88(2):227-232.
pubmed - Berntson L. Anti-inflammatory effect by exclusive enteral nutrition (EEN) in a patient with juvenile idiopathic arthritis (JIA): brief report. Clin Rheumatol. 2014;33(8):1173-1175.
doi pubmed - Smith PA. Nutritional therapy for active Crohn's disease. World J Gastroenterol. 2008;14(27):4420-4423.
doi pubmed pmc - Yamamoto T. Epidemiology of pyoderma gangrenosum in Japanese patients by questionnaire survey. J Dermatol. 2019;46(4):e145-e146.
doi pubmed - States V, O'Brien S, Rai JP, Roberts HL, Paas M, Feagins K, Pierce EJ, et al. Pyoderma Gangrenosum in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2020;65(9):2675-2685.
doi pubmed - Weizman A, Huang B, Berel D, Targan SR, Dubinsky M, Fleshner P, Ippoliti A, et al. Clinical, serologic, and genetic factors associated with pyoderma gangrenosum and erythema nodosum in inflammatory bowel disease patients. Inflamm Bowel Dis. 2014;20(3):525-533.
doi pubmed pmc - Marzano AV, Cugno M, Trevisan V, Fanoni D, Venegoni L, Berti E, Crosti C. Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin Exp Immunol. 2010;162(1):100-107.
doi pubmed pmc - Quaglino P, Fava P, Caproni M, Antiga E, De Simone C, Papini M, Parodi A, et al. Phenotypical characterization of circulating cell subsets in pyoderma gangrenosum patients: the experience of the Italian immuno-pathology group. J Eur Acad Dermatol Venereol. 2016;30(4):655-658.
doi pubmed - Oka M, Berking C, Nesbit M, Satyamoorthy K, Schaider H, Murphy G, Ichihashi M, et al. Interleukin-8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab Invest. 2000;80(4):595-604.
doi pubmed - Bister V, Makitalo L, Jeskanen L, Saarialho-Kere U. Expression of MMP-9, MMP-10 and TNF-alpha and lack of epithelial MMP-1 and MMP-26 characterize pyoderma gangrenosum. J Cutan Pathol. 2007;34(12):889-898.
doi pubmed - Menachem Y, Gotsman I. Clinical manifestations of pyoderma gangrenosum associated with inflammatory bowel disease. Isr Med Assoc J. 2004;6(2):88-90.
pubmed - Ljung T, Thomsen OO, Vatn M, Karlen P, Karlsen LN, Tysk C, Nilsson SU, et al. Granulocyte, monocyte/macrophage apheresis for inflammatory bowel disease: the first 100 patients treated in Scandinavia. Scand J Gastroenterol. 2007;42(2):221-227.
doi pubmed - Seishima M, Mizutani Y, Shibuya Y, Nagasawa C, Aoki T. Efficacy of granulocyte and monocyte adsorption apheresis for three cases of refractory pyoderma gangrenosum. Ther Apher Dial. 2007;11(3):177-182.
doi pubmed - Shanmugam VK, McNish S, Shara N, Hubley KJ, Kallakury B, Dunning DM, Attinger CE, et al. Chronic leg ulceration associated with polycythemia vera responding to ruxolitinib (Jakafi((R))). J Foot Ankle Surg. 2013;52(6):781-785.
doi pubmed pmc - Castro LGM. JAK inhibitors: a novel, safe, and efficacious therapy for pyoderma gangrenosum. Int J Dermatol. 2023;62(8):1088-1093.
doi pubmed - Ghaffari S, Kitidis C, Fleming MD, Neubauer H, Pfeffer K, Lodish HF. Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(-/-) hematopoietic progenitors. Blood. 2001;98(10):2948-2957.
doi pubmed - Mohamed MF, Beck D, Camp HS, Othman AA. Preferential Inhibition of JAK1 Relative to JAK3 by Upadacitinib: Exposure-Response Analyses of Ex Vivo Data From 2 Phase 1 Clinical Trials and Comparison to Tofacitinib. J Clin Pharmacol. 2020;60(2):188-197.
doi pubmed pmc - Peyrin-Biroulet L, Siegel C, Tanida S, Bossuyt P, Torres E, Dubinsky M, Baert F, et al. Upadacitinib promotes histologic and endoscopic mucosal healing: results from the upadacitinib ulcerative colitis phase 3 program. Journal of Crohn's and Colitis. 2022;16:i477-i478.
- Tanida S, Ozeki K, Mizoshita T, Kitagawa M, Ozeki T, Tanaka M, Nishie H, et al. Combination Therapy With Tofacitinib Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis as Induction Therapy for Refractory Ulcerative Colitis. J Clin Med Res. 2020;12(1):36-40.
doi pubmed pmc - Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7(1):48-59.
doi pubmed - Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, Thaci D, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168.
doi pubmed - Sandborn WJ, Ghosh S, Panes J, Schreiber S, D'Haens G, Tanida S, Siffledeen J, et al. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology. 2020;158(8):2139-2149.e2114.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


