| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 7, July 2023, pages 368-376
Radiologic Features of T10 Paravertebral Muscle Sarcopenia: Prognostic Factors in COVID-19
Georgios Schinasa, j, Vasiliki Dimakopouloub, j, Konstantinos Dionysopoulosc, Georgia Fezoulidid, Marianna Vlychoue, Katerina Vassiouf, Nikolaos K. Gatselisg, Anna Samakidoug, Georgios Giannoulisg, Argyrios Tzouvelekish, Markos Marangosi, Charalambos Gogosi, George N. Dalekosg, Christina Kalogeropoulouc, k, Karolina Akinosogloui, k
aSchool of Medicine, University of Patras, Patras, Greece
bDepartment of Internal Medicine, University Hospital of Patras, Patras, Greece
cDepartment of Radiology, University Hospital of Patras, Patras, Greece
dFaculty of Medicine, University of Thrace, Alexandroupolis, Greece
eDepartment of Radiology, General University Hospital of Larissa, Larissa, Greece
fDepartment of Anatomy, Medical School, University of Thessaly, Larisa, Greece
gDepartment of Medicine and Research Laboratory of Internal Medicine, Expertise Center of Greece in Autoimmune Liver Diseases, European Reference Network on Hepatological Diseases (ERN RARE-LIVER), General University Hospital of Larissa, Larissa, Greece
hDivision of Respiratory Medicine, Department of Internal Medicine, University Hospital of Patras, Patras, Greece
iDivision of Infectious Diseases, Department of Internal Medicine, University Hospital of Patras, Patras, Greece
jThese authors contributed equally to this article.
kCorresponding Author: Christina Kalogeropoulou, Department of Radiology, University Hospital of Patras, Patras, Greece; Karolina Akinosoglou, Division of Infectious Diseases, Department of Internal Medicine, University Hospital of Patras, Patras, Greece
Manuscript submitted May 28, 2023, accepted July 12, 2023, published online July 31, 2023
Short title: T10 Muscle Sarcopenia: COVID-19 Prognosis
doi: https://doi.org/10.14740/jocmr4963
| Abstract | ▴Top |
Background: Sarcopenia, defined as a small cross-sectional area (CSA) in computed tomography (CT) measurements of skeletal muscles, serves as a disease severity marker in various clinical scenarios, including pulmonary conditions and critical illness. Another parameter of sarcopenia, the level of myosteatosis, reflected by the tissue’s radiodensity, in the thoracic skeletal muscles group, has been linked to disease progression in coronavirus disease 2019 (COVID-19) patients. We hypothesize that CT-derived measurements of the skeletal muscle density (SMD) and the CSA of thoracic skeletal muscles can predict outcomes in COVID-19 pneumonia.
Methods: We retrospectively reviewed the CT scans of 84 patients with COVID-19 pneumonia admitted to two of Greece’s largest academic teaching hospitals between April 2020 and February 2021. CSA and SMD at the level of the T10 vertebra were measured using computational imaging methods. The patient population was stratified according to survival status and CT severity score (CT-SS). Correlations were drawn between the radiologic features of sarcopenia, CT severity subgroups, serum inflammatory markers, and adverse events, e.g., death and intubation.
Results: Thoracic muscles’ CSA measurements correlate with CT-SS and prominent inflammatory markers, such as white blood cell (WBC), C-reactive protein (CRP), fibrinogen, and D-dimers. Moreover, according to linear regression analysis, CSA seems to predict CT-SS variation significantly (β = -0.266, P = 0.018). CSA proved to differ significantly across survivors (P = 0.027) but not between CT severity categories and intubation subgroups. The AUC (area under the curve) of the receiver operating characteristic (ROC) curve for the predictive value of thoracic muscles’ CSA in mortality is 0.774 (95% confidence interval (CI): 0.66 - 0.83, P < 0.000). The optimal cut-off value (Youden index = 0.57) for mortality prognosis, with a sensitivity of 66.7% and a specificity of 88.9%, is 15.55. Thoracic muscles’ SMD analyses did not reveal any significant correlations.
Conclusions: Easy to obtain and accurately calculated, radiologic features can provide a reliable alternative to laboratory methods for predicting survival in COVID-19. Thoracic muscles’ CSA measurement in the level of the T10 vertebra, an acclaimed prognostic imaging assessment that relates directly to CT-SS and inflammatory markers in COVID-19 pneumonia, is a fairly specific tool for survival prognosis.
Keywords: Chest computed tomography; SAR-CoV-2; COVID-19; Prognostic factors; Cross-sectional anatomy; Muscle mass
| Introduction | ▴Top |
Chest computed tomography (CT) imaging is one of the most common modalities in assessing disease severity in coronavirus disease 2019 (COVID-19), either by conducting a conventional chest CT scan or a computed tomography pulmonary angiogram (CTPA) to evaluate both vascular and parenchymal pathology. Much information can be derived from these fast and straightforward imaging procedures that their use has become invaluable during the pandemic, especially in patients with critical disease, that may require immediate interventions or intensive monitoring. For these purposes, the COVID-19 CT severity score (CT-SS) has been established as a prognostic tool for mortality and severity [1]. At the same time, body composition and metabolic factors have become more relevant than ever in predicting outcomes in COVID-19 [2].
Sarcopenia, defined as the loss of lean muscle mass due to aging, is a relatively new pathologic entity, receiving an International Classification of Diseases, 10th Revision (ICD-10) classification code only as recently as 2016 [3]. According to the latest guidelines, sarcopenia is determined by both low muscle quantity and quality [4]. Intuitively, muscle mass and its quality, in terms of density, may represent the amount of metabolic reserves of a human organism in order to withstand the stress imposed by disease. Sarcopenia has been linked with poor outcomes in critically ill patients [5]. Reportedly, sarcopenia is an established risk factor for various pulmonary conditions, including the development of pneumonia in older adults and chronic obstructive pulmonary disease (COPD) exacerbations [6].
CT-derived measurements such as skeletal muscle cross-sectional area (CSA) and attenuation measured in Hounsfield unit (HU), represent surrogates for skeletal muscle mass/area (CSA) and skeletal muscle density (SMD), respectively, hence, can be used for the assessment of sarcopenia. In fact, these measurements are routinely used as prognostic markers in patients with malignancy and COPD [7-9]. A variety of muscle groups have been proposed for assessing the prognostic value of their respective CSA and attenuation levels in different settings. Reduced quantitative and qualitative measurements of the T12 paravertebral muscle group indicate an unfavorable course of disease in older adult patients with COPD exacerbation [6], lung transplant recipients [10], or idiopathic pulmonary fibrosis [11].
Up to the present, the impact of sarcopenia in COVID-19 patients has not been thoroughly assessed. Several studies have evaluated the association between CT-muscle analysis of sarcopenia and COVID-19 patients, suffering distinct variation in the level of radiologic assessment, employed muscle groups, and proposed thoracic cut-off values for sarcopenia [12]. Patients with low skeletal mass as determined by CT-derived cross-sectional imaging are more likely to have poor outcomes when hospitalized for COVID-19 [13]. For instance, measurements of pectoralis have been well documented to predict outcomes and disease severity in COVID-19 [14].
Therefore, we chose to focus on the paravertebral muscles at the lowest possible vertebral level, as captured on the chest CT scan upon admission, in an attempt to compensate for body composition variance and height inequality, since the CSA of the paravertebral muscles is not influenced as much by anthropometrics and physical activity [15]. Paraspinal muscles at the level of the T10 vertebra were the lowest-level muscle group best visualized on CT. On these grounds, we aimed to explore whether paravertebral muscle area as measured in the CT-scan CSA at the level of the T10 vertebra and the attenuation level of the same muscle group, at the time of admission can predict the outcome of COVID-19 patients and correlate with established markers of the inflammatory process and disease severity.
| Materials and Methods | ▴Top |
Study population
This was a two-center retrospective observational study conducted in two COVID-19 referral teaching hospitals between April 2020 and February 2021. Our study was conducted in accordance with the declaration of Helsinki and principles of good clinical practice and was approved by local institutional review boards and ethics committee (268/10.05.2021). Two teams of experienced radiologists, blinded to the outcomes and relative clinical information, reviewed the chest CT scans of adult patients admitted to the two hospitals with a confirmed diagnosis of COVID-19-related pneumonia, as evidenced by a positive result on a reverse transcriptase-polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab and pertinent radiological findings. Only patients who had undergone a chest CT scan or a CTPA upon admission, prior to initiating any standard COVID-19 treatment, and had electronically stored CT images that were suitable for analysis, were included in the study. Exclusion criteria encompassed patients unable to undergo a CT scan due to their unstable condition, those with a history of inherited/acquired neuromuscular and/or degenerative diseases, feeding disorders or nutritional deficiencies or poorly controlled endocrine disorders, and those who had received treatments that could affect muscle mass, such as corticosteroids or chemotherapy, within the past 6 months. Patients with incomplete medical records, missing data on key variables, pregnant women, and patients transferred from other hospitals were also excluded from the study.
Measurement of T10-CSA and T10-SMD
The electronically stored CT images of all patients (either chest CTs or CTPAs) performed within 48 h post admission were analyzed using Picture Archiving and Communication Systems (PACS). The CSA at the T10 vertebral level (T10-CSA) was defined as any muscle within the region posterior to the T10 spine and ribs and no more lateral than the lateral-most edges of the erector spinal muscles (Fig. 1) [16].
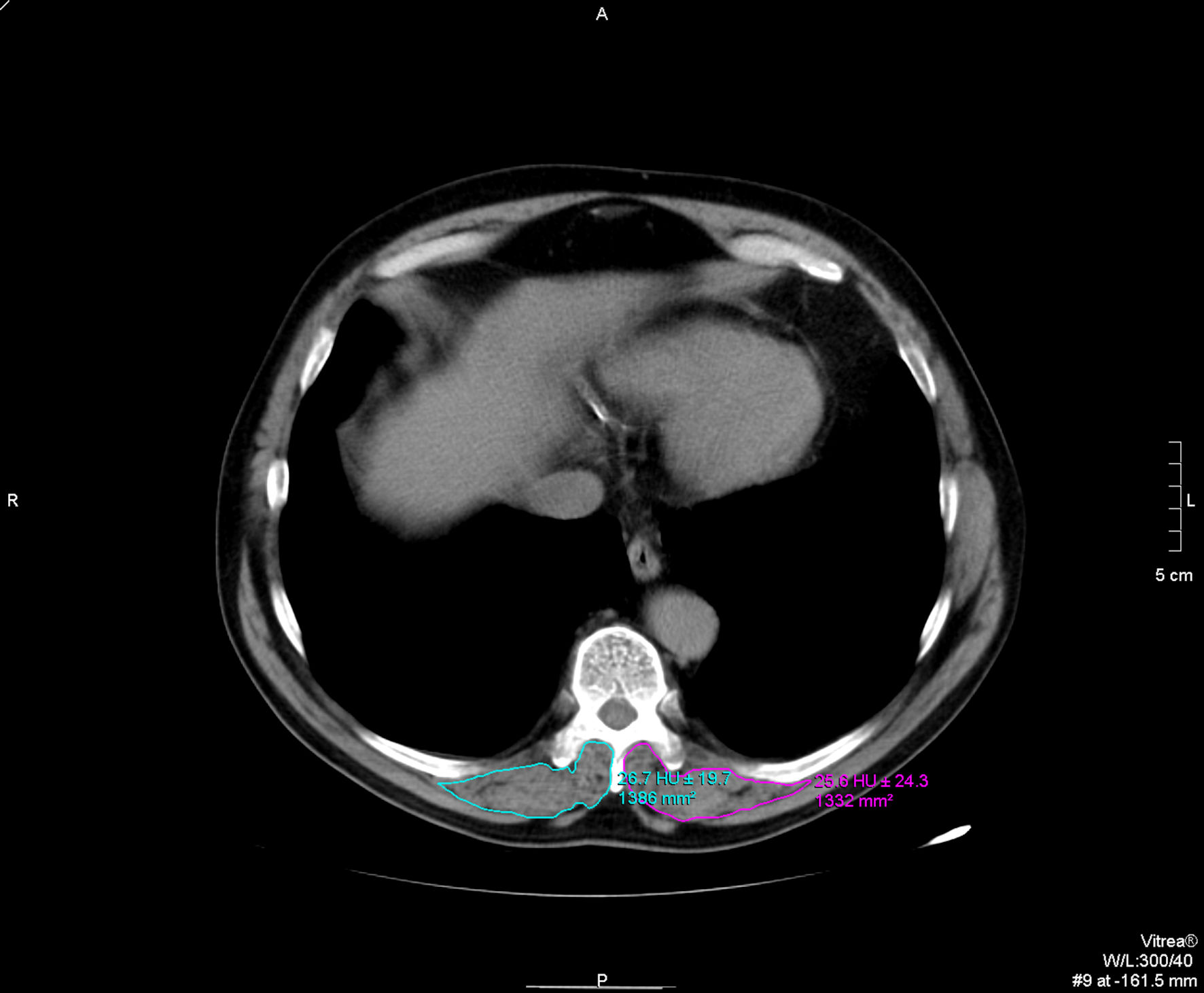 Click for large image | Figure 1. T10 paravertebral muscle group cross-sectional area (CSA) and skeletal muscle density (SMD) measurements. |
The T10-CSA was used as an alternative to T12-CSA [6] as in some patients the T12 level was not included in the CT images. All imaging analyses were conducted in the axial plane at the level of the 10th thoracic spine, and manual sketch ridge of the CSA of the dorsal muscle were performed for each side. T10- CSA was recorded as the sum of bilateral dorsal muscle area in cm2, while T10-SMD was recorded as the mean of bilateral dorsal muscle radiodensity in HU. The measurements of T10-CSA and T10-SMD were independently performed in each center, by two experienced radiologists and the final measurements were conjointly agreed upon.
Measurement of CT-SS
Using lung windows of the CT images the extend of the lung parenchymal involvement was also evaluated, and the CT-SS was calculated by using a semiquantitative scoring system [17, 18]. The total CT-SS ranged from 0 (no involvement) to 25 (maximum involvement), and CT severity was defined as mild for a score of 7 or less, moderate for a score of 8 - 17 and severe for a score of 18 - 25 [1, 17, 18]. All CT images were reviewed using PACS. Patients’ charts were then independently reviewed and demographic, clinical and laboratory parameters, as well as outcome measures extracted.
Statistical analysis
All quantitative variables are described as the median and interquartile range (IQR). All qualitative variables are described as percentages. The Shapiro-Wilk test was used to assess normality across all variables’ distribution. The independent-samples t-test was performed for comparison of normally distributed variables. Mann-Whitney U test was the non-parametric test of choice for the rest of variables. Potential correlations between pertinent inflammatory values and sarcopenia values were examined using Spearman’s Rho. Linear and ordinal regression analyses were conducted to evaluate how CSA and attenuation measurements relate to the CT-SS and severity subgroups. In order to investigate the predictive value of the model, area under the curve (AUC) was calculated, and the Youden index was obtained so to establish the optimal cut-off values. We utilized binary logistic regression to examine the association between the two radiological values and survival. To ensure the robustness of our multivariate analysis, we adopted a systematic approach for variable selection, aiming to include only the most pertinent predictors of the outcome in the final model. Initially, we performed a comparative analysis followed by univariate analyses to identify potential predictors. Variables that demonstrated a significant association with the outcome in the univariate analyses were considered for inclusion in the multivariate model. Given the multiple comparisons made, we implemented a Bonferroni adjustment to reduce the likelihood of type I errors (false positives). Consequently, a more stringent significance level of P < 0.02 was set for inclusion in the model. Two-tailed P values of less than 0.05 were considered significant for the rest of the analysis.
| Results | ▴Top |
The results of this study are based on 84 chest CT scans of patients who were diagnosed with COVID-19. The demographic, clinical, and laboratory characteristics of the subjects are presented in Table 1. A total of 59 (70%) of the subjects were male, and the median age was 60 (IQR: 47 - 71) years. Eighteen percent of the patients required mechanical ventilation (MV) during their hospitalization, while 21% of the subjects died from the disease. The median time from symptom onset to hospital admission was 7 days (IQR: 4 - 8), with no significant difference between survivors and deceased patients (P = 0.58). The prevalence of comorbidities such as coronary artery disease (CAD), hypertension, type 2 diabetes mellitus, dyslipidemia, and other metabolic comorbidities did not significantly differ between survivors and deceased patients.
 Click to view | Table 1. Study Population Characteristics |
The median CT-SS of all subjects was 10 (IQR: 6.25 - 15) and showed a significant difference between survivors (P < 0.001). The inflammatory values were assessed at baseline, and only lactate dehydrogenase (LDH) was found to be significantly different between the two groups, with a value of 260 (IQR: 181 - 338) U/L in the survived group and 319 (IQR: 255 - 377) U/L in the deceased group (P = 0.022). The median albumin level was also significantly lower in deceased patients (3.3 g/dL, IQR: 2.95 - 3.98) compared to survivors (4.4 g/dL, IQR: 3.99 - 4.62) (P = 0.005).
The T10-CSA was significantly lower in the deceased group (11.91 cm2, IQR: 8.85 - 14.498) compared to the alive group (18.24 cm2, IQR: 12.845 - 25.445) (P < 0.001), while no difference was found in SMD between the two groups. CSA was negatively correlated with CT-SS (r = -0.239, P = 0.028), white blood cell count (WBC) (r = -0.287, P = 0.012), fibrinogen (r = -0.3921, P = 0.001), D-dimers (r = -0.363, P = 0.003), C-reactive protein (CRP) (r = -0.315, P = 0.006).
Simple linear regression revealed that T10-CSA significantly predicted CT-SS, with a decrease of 0.21 for each cm2 increase in CSA (R-squared = 0.73, P = 0.013). In univariate analysis, T10-CSA was significantly associated with survival (odds ratio (OR) = 1.195, 95% confidence interval (CI): 1.07 - 1.33, P = 0.002). In a multivariable model, accounting for patients’ age, CT-SS, LDH, and need for MV, T10-CSA was found to be an independent predictor of hospital discharge (adjusted OR = 1.207; 95% CI: 1.004 - 1.45, P = 0.045). The unadjusted ORs for hospital discharge and the ORs adjusted for all variables included in the final model are presented in Table 2.
 Click to view | Table 2. Logistic regression results on the impact of each variable on the odds of survival |
To further investigate the predictive value of T10-CSA on survival, the receiver operating characteristic (ROC) curve was plotted. The AUC of the ROC was 0.774 (95% CI: 0.66 - 0.83, P < 0.001) (Fig. 2). The optimal cut-off value for prognosis of survival, calculated using the Youden index, was 15.55 cm2, with a sensitivity of 66.7% and specificity of 88.9%. The diagnostic accuracy of T10-CSA is presented in a flow chart according to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines in Figure 3.
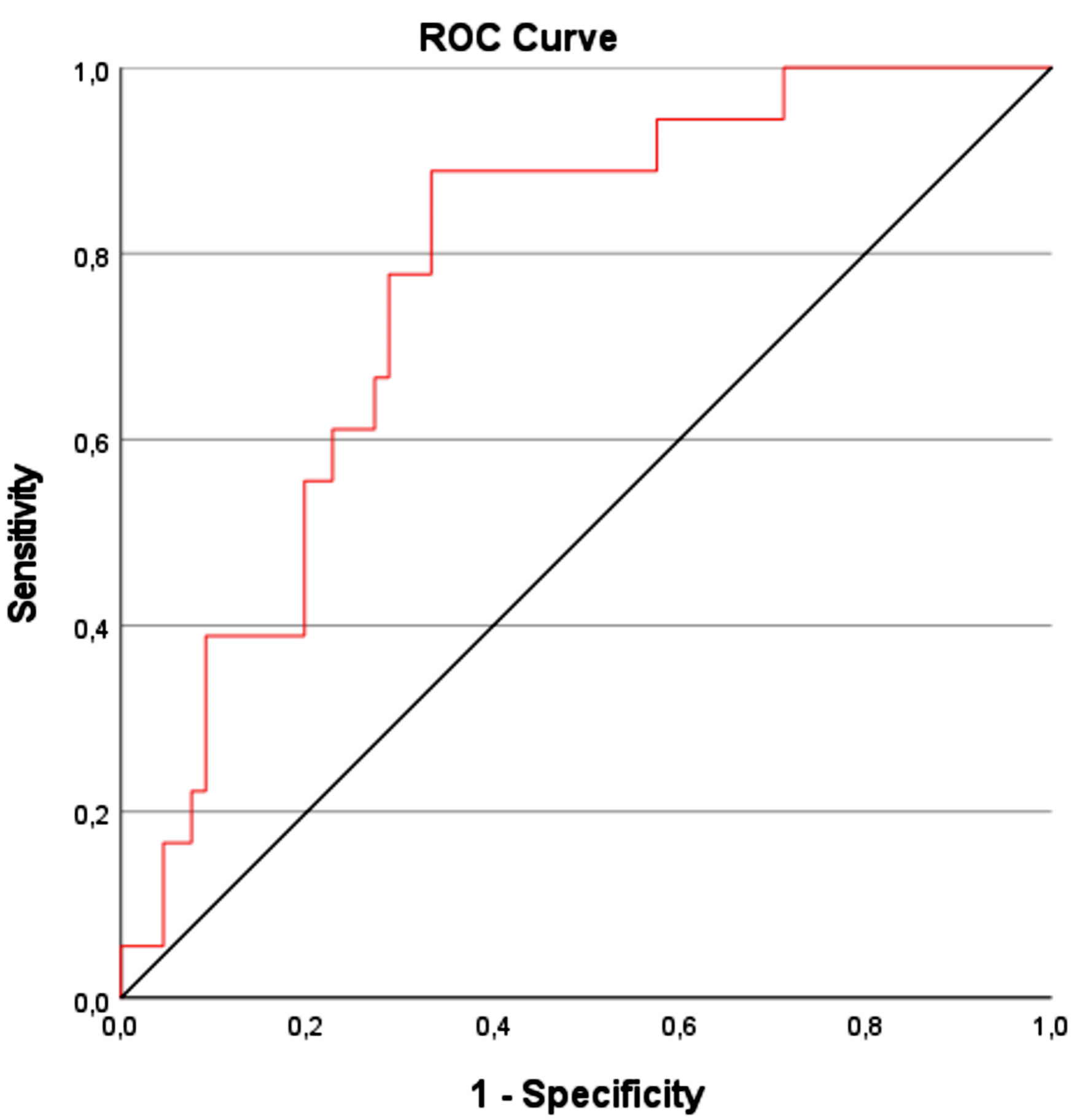 Click for large image | Figure 2. Plotted ROC curve of T10-CSA for hospital survival prediction. ROC: receiver operating characteristic; CSA: cross-sectional area. |
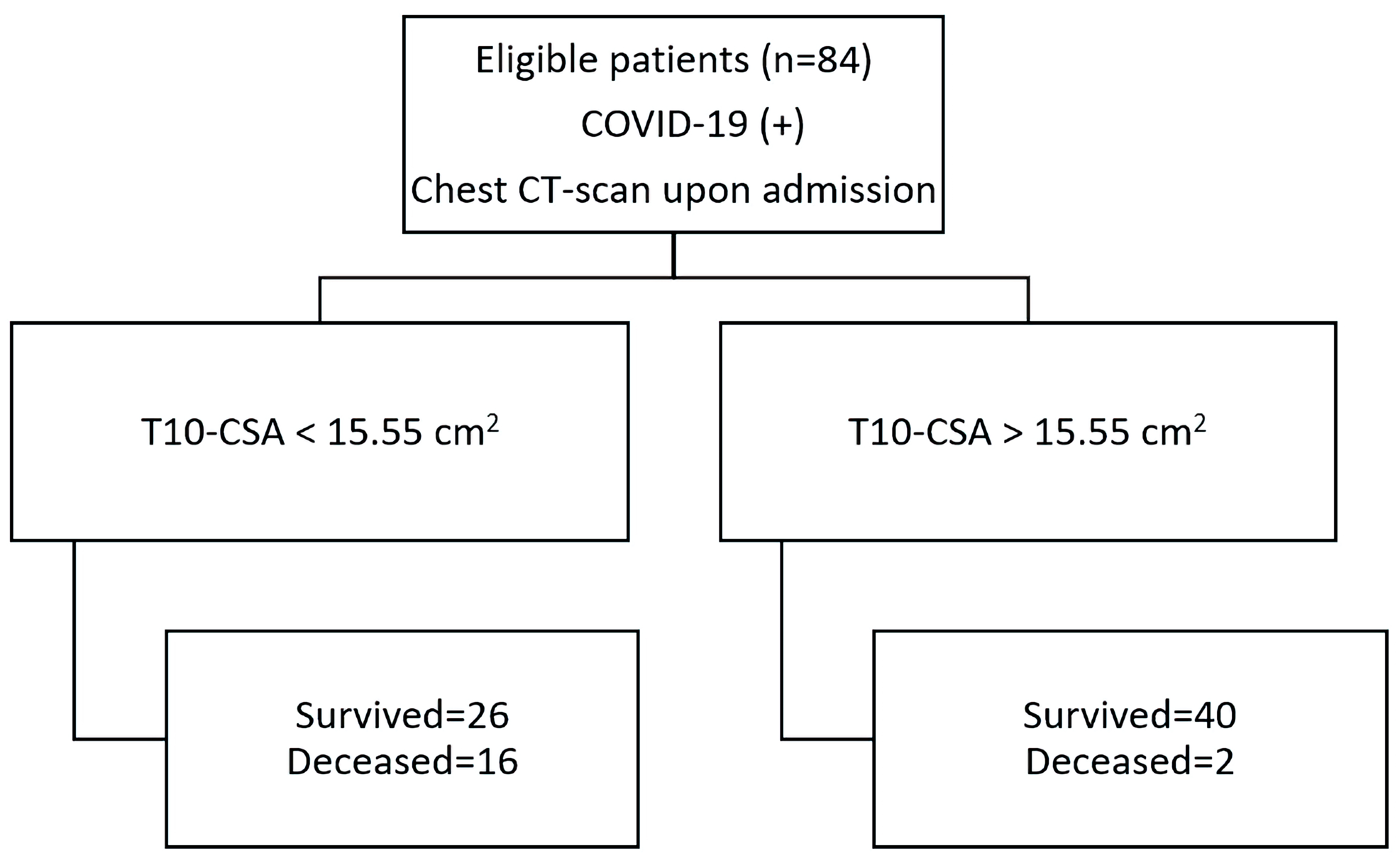 Click for large image | Figure 3. T10-CSA diagnostic accuracy flow chart. COVID-2019: coronavirus disease 2019; CSA: cross-sectional area. |
| Discussion | ▴Top |
Following 2.5 years in COVID-19 pandemic, and despite clinicians’ expanded knowledge in managing patients with COVID-19-related pneumonia, medical systems worldwide still struggle to keep up with the increasing number of cases and the sheer volume of patients requiring hospitalization. Recent epidemiological reports point to the fact that a better qualitative assessment of the patient’s condition is needed to tackle increasing mortality, especially in cases that will eventually require intensive care unit (ICU) monitoring [19].
One of the strategies employed to overcome the logistical difficulties posed by the number of hospitalizations is establishing prognosis early in the course of the disease using accurate biomarkers to distribute patients appropriately and allocate resources accordingly. A number of serum prognostic biomarkers have been explored in order to drive therapeutic modalities, involving variety of pathophysiological mechanisms including inflammation and/or tissue damage [20, 21]. However, the assay methods, cutoffs, time points of measurement, as well as availability of otherwise well described biomarkers in respective reports remain variable.
The potential biomarkers should be easy to obtain and subjective to interpret. Calculative radiologic features are optimal in both these respects, as they are readily available upon request and relatively independent of operator bias and expertise. Radiomic analyses of tomographic images may be the next frontier in clinical decision-making and precision medicine [22]. Previous reports have described a number of radiographic features facilitating prognosis and response monitoring, including qualitative and quantitative radiomic features of density and damage extent in both chest X-rays and CT scans [23-25].
Despite our study population exhibiting a relatively low level of underlying inflammation, as indicated by the mild elevations in inflammatory markers and the brief duration of disease prior to hospital admission, T10-CSA correlated well with inflammatory markers, such as WBC count, CRP, fibrinogen, and D-dimers. This is not surprising, considering that some of the most prominent inflammatory pathways in the development of sarcopenia, including systemic inflammation and tissue hypoxia leading to oxidative stress, are also involved in COVID-19 [26]. In line with the previous findings, T10-CSA showed good association with CT-SS, hence disease severity. In our study group analysis, CT-SS but not CSA, correlated firmly with the length of stay and various inflammatory markers. This comes in contrast with other reports, which showed that measurement of CSA of the pectoralis muscle area (PMA) was an independent predictor of length of stay [27].
Several - nonetheless small reports - have confirmed the role of sarcopenia in patients’ outcome, even though the level of radiologic assessment, employed muscle groups and proposed thoracic cut-off values for sarcopenia detection vary among studies. Some authors have even utilized thoracic CT-derived measurements to predict the degree of abdominal sarcopenia and correlate that to poor outcomes [28, 29]. Data derived from intubated patients have shown that patients with shorter ICU stay and successful extubation had higher PMA and pectoralis muscle density (PMD) compared to those with longer stay, as this measured at the T4 level [30]. This could be partly explained by the fact that sarcopenia impairs the ability to produce adequate respiratory volumes after MV; thus, causing a slower recovery of spontaneous breathing [31]. However, no statistical difference in PMA and PMD was noted regarding CT severity pneumonia as observed in our study. It is possible that CT severity strictly depends on the time it is performed with possible sudden clinical and radiological worsening, hence associations could only be detected later in the course of disease. In agreement with previous reports, Damanti et al showed that muscle mass is associated with successful extubation and shorter ICU stay, while lower muscle density is associated with a longer hospitalization and in hospital mortality when measured in L1 - L3 levels [32]. In a larger cohort of a CT-based model of 552 COVID-19 patients with CTs performed on emergency departments during admission, lower-than-median T5 paravertebral muscle area was independently associated with ICU admission (OR: 4.3, P < 0.001) and hospital mortality (OR: 2.3, P = 0.01) [33]. In their study authors proposed a combined model of CT-derived muscle status and lung disease extent that allowed to predict death (AUC: 0.81) [33]. In addition, Kim et al demonstrated that patients with sarcopenia had increased time to discharge and a higher incidence of death than those without sarcopenia, as this measured at T12 level [34]. Interestingly, the association between baseline sarcopenia and delayed hospital discharge was consistent in subgroups stratified by age, sex, comorbidities, and severity of COVID-19 [34]. Finally, in a French cohort studying the predictive potential of thoracic muscle sarcopenia, that was conducted during the first wave of the pandemic, decreased thoracic skeletal muscle index (SMI) at the T12 level strongly associated with ICU admission (OR = 5.56, P < 0.05) and correlated with prolonged length of stay as well as newly acquired infections. In contrast to our results, however, no significant association was detected in terms of overall survival [35].
SMD at the T10 level proved of no value, neither in terms of survival nor of prediction of severity in our study. Moreover, our analysis did not reveal any correlation with the inflammatory markers, except for LDH. Nonetheless, a report from China suggests that thoracic muscle steatosis at the level of the T10 vertebra may predict transition to severe COVID-19 [36]. Finally, our observation that albumin levels were significantly different among survivor groups may initially seem to provide a pathophysiological basis for our findings. However, it is crucial to understand that this does not necessarily imply a causal relationship with pre-existing sarcopenia, as albumin levels typically decrease in conditions characterized by systemic inflammation and catabolism, both of which are prevalent in COVID-19. This observation aligns with findings from other studies, which have identified a correlation between low serum albumin levels upon admission and increased severity of COVID-19, as well as higher rates of adverse outcomes [37, 38].
Our study has several limitations that must be taken into consideration when interpreting the results. First and foremost, our study is retrospective in nature and only includes a limited sample size. Given the novelty of our investigation and the lack of prior studies on which to base an estimation of effect size, it was challenging to determine an appropriate sample size a priori. This may have introduced some bias into our patient selection and affected the generalizability of our findings. Additionally, our patient population was sampled across two different centers, which may have led to differences in computed attenuation values between CT machines and patient positioning.
Furthermore, patients with critical disease who were unable to undergo a CT scan due to their unstable condition were naturally excluded from the sample. This may have led to an underestimation of the severity of sarcopenia in critically ill patients. Our study was adequately powered (β = 0.2, α = 0.05) to detect a 25% decrease in CSA, however, the sample size was still relatively small and may have limited the ability to detect smaller differences in CSA or SMD.
In addition, only a few female subjects were included in this study, which may affect the ability to generalize the results to female patients with COVID-19. The male prevalence in the mortality group, however, is depicted accurately for this age group [34]. Age, a non-modifiable independent risk factor for COVID-19 mortality [35], also differed significantly across survival groups. Sarcopenia being influenced by both physiologic and acute stress, is an inadvertently age-related process [36].
Moreover, the absence of a control group also makes it difficult to establish a causal relationship between T10-CSA and survival outcomes in patients with COVID-19. Given the exploratory nature of our study, we focused on investigating the potential of T10-CSA as a prognostic marker at admission, a single-point in time. We acknowledge the absence of model validation (external or internal) to be a crucial limitation in ensuring the reliability and generalizability of our results. Overall, these limitations highlight the need for further longitudinal studies to confirm our findings and explore the relationship between sarcopenia and survival outcomes in COVID-19 over time.
Conclusions
Unfortunately, pharmacologic options for tackling sarcopenia do not exist, although therapeutic targets have been established and new drugs are in development [39]. At the moment sarcopenia can only be utilized for prognostic purposes, in order to prompt quick responses. Our results demonstrate that paravertebral muscle CSA measurements at the level of the T10 vertebra upon hospital admission yield predictive value in determining survival. T10-CSA’s relatively high specificity as a prognostic marker may help distinguish patients with severe disease that could benefit from an increased level of care. T10-CSA is an easy to acquire radiologic measurement and a relatively specific marker of hospital survival. Further studies into its predictive value should be conducted, especially in large prospective cohorts of patients, to verify our results.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author Contributions
KA, CK, and MV conceptualized study. GS, VD, GF, KV, AS, and GG collected data. KD, MV, and CK performed radiologic measurements. GS and KA analyzed data. AT, MM CG, CK, and KA oversaw study. GS and KA wrote the manuscript and drew figures and tables. KA, CK, GD, and NKG critically corrected manuscript. GS and KA revised manuscript.
Data Availability
Data are available upon reasonable request from the corresponding author.
| References | ▴Top |
- Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q, Luo Y, et al. Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging. 2020;2(2):e200047.
doi pubmed pmc - Chandarana H, Pisuchpen N, Krieger R, Dane B, Mikheev A, Feng Y, Kambadakone A, et al. Association of body composition parameters measured on CT with risk of hospitalization in patients with Covid-19. Eur J Radiol. 2021;145:110031.
doi pubmed pmc - Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J Am Med Dir Assoc. 2016;17(8):675-677.
doi pubmed - Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601.
doi pubmed pmc - Kizilarslanoglu MC, Kuyumcu ME, Yesil Y, Halil M. Sarcopenia in critically ill patients. J Anesth. 2016;30(5):884-890.
doi pubmed - Zhi J, Shan Q, Liang L, Liu H, Huang H. Low skeletal muscle area as a prognostic marker for chronic obstructive pulmonary disease in elderly patients admitted to ICU. Sci Rep. 2019;9(1):19117.
doi pubmed pmc - Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839.
doi pubmed - Buentzel J, Heinz J, Bleckmann A, Bauer C, Rover C, Bohnenberger H, Saha S, et al. Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res. 2019;39(9):4603-4612.
doi pubmed - Benz E, Trajanoska K, Lahousse L, Schoufour JD, Terzikhan N, De Roos E, de Jonge GB, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28(154):1-13.
doi pubmed pmc - Rozenberg D, Wickerson L, Singer LG, Mathur S. Sarcopenia in lung transplantation: a systematic review. J Heart Lung Transplant. 2014;33(12):1203-1212.
doi pubmed - Moon SW, Choi JS, Lee SH, Jung KS, Jung JY, Kang YA, Park MS, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res. 2019;20(1):35.
doi pubmed pmc - Xu Y, Xu JW, You P, Wang BL, Liu C, Chien CW, Tung TH. Prevalence of sarcopenia in patients with COVID-19: a systematic review and meta-analysis. Front Nutr. 2022;9:925606.
doi pubmed pmc - Meyer HJ, Wienke A, Surov A. Computed tomography-defined body composition as prognostic markers for unfavourable outcomes and in-hospital mortality in coronavirus disease 2019. J Cachexia Sarcopenia Muscle. 2022;13(1):159-168.
doi pubmed pmc - Hocaoglu E, Ors S, Yildiz O, Inci E. Correlation of Pectoralis Muscle Volume and Density with Severity of COVID-19 Pneumonia in Adults. Acad Radiol. 2021;28(2):166-172.
doi pubmed pmc - Gibbons LE, Videman T, Battie MC, Kaprio J. Determinants of paraspinal muscle cross-sectional area in male monozygotic twins. Phys Ther. 1998;78(6):602-610; discussion 611-602.
doi pubmed - Lee CS, Cron DC, Terjimanian MN, Canvasser LD, Mazurek AA, Vonfoerster E, Tishberg LM, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant. 2014;28(10):1092-1098.
doi pubmed pmc - Saeed GA, Gaba W, Shah A, Al Helali AA, Raidullah E, Al Ali AB, Elghazali M, et al. Correlation between Chest CT severity scores and the clinical parameters of adult patients with COVID-19 pneumonia. Radiol Res Pract. 2021;2021:6697677.
doi pubmed pmc - Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715-721.
doi pubmed pmc - Lytras T, Tsiodras S. Total patient load, regional disparities and in-hospital mortality of intubated COVID-19 patients in Greece, from September 2020 to May 2021. Scand J Public Health. 2022;50(6):671-675.
doi pubmed - Papadopoulou G, Manoloudi E, Repousi N, Skoura L, Hurst T, Karamitros T. Molecular and clinical prognostic biomarkers of COVID-19 severity and persistence. Pathogens. 2022;11(3):311.
doi pubmed pmc - Bivona G, Agnello L, Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021;41(6):540-548.
doi pubmed pmc - Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, They Are Data. Radiology. 2016;278(2):563-577.
doi pubmed pmc - Ferreira Junior JR, Cardona Cardenas DA, Moreno RA, de Sa Rebelo MF, Krieger JE, Gutierrez MA. Novel chest radiographic biomarkers for COVID-19 using radiomic features associated with diagnostics and outcomes. J Digit Imaging. 2021;34(2):297-307.
doi pubmed pmc - Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296(2):E115-E117.
doi pubmed pmc - Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, Xie RM, et al. Imaging Features of Coronavirus disease 2019 (COVID-19): Evaluation on Thin-Section CT. Acad Radiol. 2020;27(5):609-613.
doi pubmed pmc - Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21(4):543-559.
doi pubmed pmc - Ufuk F, Demirci M, Sagtas E, Akbudak IH, Ugurlu E, Sari T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur J Radiol. 2020;131:109271.
doi pubmed pmc - Molwitz I, Ozga AK, Gerdes L, Ungerer A, Kohler D, Ristow I, Leiderer M, et al. Prediction of abdominal CT body composition parameters by thoracic measurements as a new approach to detect sarcopenia in a COVID-19 cohort. Sci Rep. 2022;12(1):6443.
doi pubmed pmc - Koehler J, Boirie Y, Bensid L, Pereira B, Ghelis N, Dupuis C, Tournadre A, et al. Thoracic sarcopenia as a predictive factor of SARS-COV2 evolution. Clin Nutr. 2022;41(12):2918-2923.
doi pubmed pmc - Antonarelli M, Fogante M. Chest CT-derived muscle analysis in COVID-19 patients. Tomography. 2022;8(1):414-422.
doi pubmed pmc - Ali AM, Kunugi H. Screening for Sarcopenia (Physical Frailty) in the COVID-19 Era. Int J Endocrinol. 2021;2021:5563960.
doi pubmed pmc - Damanti S, Cristel G, Ramirez GA, Bozzolo EP, Da Prat V, Gobbi A, Centurioni C, et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin Nutr. 2022;41(12):2965-2972.
doi pubmed pmc - Schiaffino S, Albano D, Cozzi A, Messina C, Arioli R, Bna C, Bruno A, et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. 2021;300(2):E328-E336.
doi pubmed pmc - Kim JW, Yoon JS, Kim EJ, Hong HL, Kwon HH, Jung CY, Kim KC, et al. Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2021;76(8):e110-e116.
doi pubmed pmc - Grigioni S, Lvovschi VE, Tamion F, Joly LM, Coeffier M, Van Elslande H, Galmiche M, et al. Low thoracic skeletal muscle index is associated with negative outcomes in 244 patients with respiratory COVID-19. Clin Nutr. 2023;42(2):102-107.
doi pubmed pmc - Yi X, Liu H, Zhu L, Wang D, Xie F, Shi L, Mei J, et al. Myosteatosis predicting risk of transition to severe COVID-19 infection. Clin Nutr. 2022;41(12):3007-3015.
doi pubmed pmc - Kheir M, Saleem F, Wang C, Mann A, Chua J. Higher albumin levels on admission predict better prognosis in patients with confirmed COVID-19. PLoS One. 2021;16(3):e0248358.
doi pubmed pmc - Turcato G, Zaboli A, Kostic I, Melchioretto B, Ciccariello L, Zaccaria E, Olivato A, et al. Severity of SARS-CoV-2 infection and albumin levels recorded at the first emergency department evaluation: a multicentre retrospective observational study. Emerg Med J. 2022;39(1):63-69.
doi pubmed pmc - Dhillon RJ, Hasni S. Pathogenesis and Management of Sarcopenia. Clin Geriatr Med. 2017;33(1):17-26.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


