| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 7, July 2023, pages 343-359
Protein Induced by Vitamin K Absence or Antagonist-II Versus Alpha-Fetoprotein in the Diagnosis of Hepatocellular Carcinoma: A Systematic Review With Meta-Analysis
Abdallah Kobeissya, h, Nooraldin Merzab, Alsadiq Al-Hillanc, Safa Boujemaad, Zohaib Ahmedb, Mohamad Nawrase, Mohammed Albaajf, Dushyant Singh Dahiyag, Yaseen Alastala, Mona Hassana
aDepartment of Gastroenterology, The University of Toledo, Toledo, OH, USA
bDepartment of Internal Medicine, The University of Toledo, Toledo, OH, USA
cGastroenterology Department, Corewell Health/Willam Beaumont University Hospital, Royal Oak, MI, USA
dBiotechnology Development, Institute Pasteur De Tunis, Universite De Tunis El Manar, Tunis, Tunisia
eThe University of Toledo, College of Medicine and Life Sciences, Toledo, OH, USA
fDepartment of Pharmacology and Experimental Therapeutics, University of Toledo, Toledo, OH, USA
gDivision of Gastroenterology, University of Kansas School of Medicine, Kansas City, KS, USA
hCorresponding Author: Abdallah Kobeissy, Department of Gastroenterology, The University of Toledo, Toledo, OH, USA
Manuscript submitted May 13, 2023, accepted June 9, 2023, published online July 12, 2023
Short title: PIVKA-II vs. AFP in the Diagnosis of HCC
doi: https://doi.org/10.14740/jocmr4951
| Abstract | ▴Top |
Background: Protein induced by vitamin K absence or antagonist-II (PIVKA-II) and α-fetoprotein (AFP) are promising tumor markers for the diagnosis of hepatocellular carcinoma (HCC). Yet, their diagnostic performance differs throughout HCC investigations. The aim of this meta-analysis was to assess the effectiveness of PIVKA-II and AFP in the diagnosis of HCC.
Methods: A systematic literature search was performed to identify relevant studies from eight databases, which were published up to February 2023, in order to compare the diagnostic performance of PIVKA-II and AFP for HCC. Pooled sensitivity and specificity were calculated. Summary receiver operating characteristic (SROC) curve was performed to assess the diagnostic accuracy of each biomarker.
Results: Fifty-three studies were identified. The pooled sensitivity (95% confidence interval (CI)) of PIVKA-II and AFP was 0.71 (0.70 - 0.72) and 0.64 (0.63 - 0.65), respectively in diagnosis of HCC, and the corresponding pooled specificity (95% CI) was 0.90 (0.89 - 0.90) and 0.87 (0.87 - 0.88), respectively. The area under the ROC curve (AUC) of PIVKA-II and AFP was 0.89 (0.88 - 0.90) and 0.78 (0.77 - 0.79), respectively. Subgroup analysis demonstrated that PIVKA-II presented higher AUC values compared to AFP in terms of ethnic group (African, European, Asian, and American patients), etiology (mixed-type HCC, hepatitis C virus (HCV)-related, and hepatitis B virus (HBV)-related) and sample size of cases (≤ 100 and > 100).
Conclusion: This study reveals that PIVKA-II is a promising biomarker for identifying and tracking HCC, exhibiting greater accuracy than AFP. Our findings indicate that PIVKA-II outperforms AFP in detecting HCC across diverse racial groups and sample sizes, as well as in cases of HBV-related, HCV-related, or mixed-etiology HCC.
Keywords: Vitamin K absence or antagonist-II; Alpha-fetoprotein; Hepatocellular carcinoma; Hepatitis B virus; Hepatitis C virus
| Introduction | ▴Top |
Liver cancer accounts for 841,000 new cases and 782,000 fatalities annually, making it the sixth most prevalent cancer overall [1]. In all primary liver malignancies, hepatocellular carcinoma (HCC) accounts for more than 90% of cases. It is still becoming more common, ranking as the second most common reason for cancer-related deaths [2]. The most frequent etiological causes of HCC include alcoholic liver disease, non-alcoholic fatty liver disease, and hepatitis B virus (HBV) or hepatitis C virus (HCV) infection [3]. The early stages of HCC are typically asymptomatic and have a propensity for intravascular and invasive behavior. Due to a lack of efficient diagnostic methods, more than two-thirds of patients do not receive a diagnosis until an advanced stage [4]. The prognosis of HCC varies significantly depending on the tumor stage at the time of diagnosis; as a result, early detection is essential to enable therapeutic methods and hence increase patient survival [5]. Presently, abdominal ultrasonography (US) is the mainstay of monitoring programs for HCC diagnosis in high-risk populations. The US is a non-invasive and cost-effective screening approach. However, it has sub-optimal sensitivity [6]. Moreover, the presence of a coarse liver echo pattern, obesity, meteorism, chest wall abnormalities, prior abdominal surgery, a lack of patient participation, and the operator’s skill are some other factors that may affect the US examination [7]. As a result, alternative non-invasive techniques must be used in conjunction with the US to enhance the detection of possible malignant tumors such as serum biomarkers. However, the use of serum biomarkers is still up for debate and lack of trustworthy blood biomarkers is, in fact, a serious problem with HCC surveillance [6]. Alpha-fetoprotein (AFP) has received the most attention among non-invasive biomarkers and has been shown to have a diagnostic accuracy of 0.767 (0.732 - 0.803) for HCC diagnosis [8]. AFP-L3, an AFP variant, holds significant importance in diagnosing HCC. AFP-L3 is a specific isoform of AFP associated with malignant liver tumors, particularly HCC. It provides higher specificity than total AFP, making it a valuable biomarker for detecting and differentiating HCC from other liver diseases early [8-10].
In the same context, Trevisani et al revealed that AFP showed 60-80% sensitivity at a cut-off value of 20 ng/mL [9]. However, data on the effectiveness of AFP combined with US in a surveillance situation are still inconclusive [10].
Also known as “des-gamma-carboxy prothrombin” (DCP), protein induced by vitamin K absence or antagonist-II (PIVKA-II) is a prothrombin protein that is aberrant as a result of an acquired deficiency in the post-translational carboxylation of the prothrombin precursor in cancerous cells [11]. Several research articles demonstrated that PIVKA-II presents a high sensitivity (up to 90%) and specificity (up to 100%) at distinguishing HCC from other chronic liver disorders [11-13]. Many studies have compared the diagnostic value of PIVKA-II and AFP, but findings are conflicting and, data about whether PIVKA-II and AFP perform differently in diagnosing HCC of different etiologies should be updated.
Therefore, this systematic review and meta-analysis was conducted to provide up-to-date evidence on the diagnostic performance of PIVKA-II and AFP for the detection of HCC.
| Materials and Methods | ▴Top |
Search strategy
This systematic review and meta-analysis study was carried out; the work has been reported in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines [14]. A systematic search was conducted on PubMed, Medline, Web of Science, Scopus, the Cochrane Library, Embase, Google Scholar, and CINAHL from database inception until February 2023 to look for potentially eligible articles. The search strategy used the following combinations of search terms: “des-gamma-carboxy prothrombin” OR “protein induced by vitamin K absence or antagonist II” OR “PIVKA-II” OR “DCP” AND “alpha-fetoprotein” OR “AFP” AND “hepatocellular carcinoma” OR “primary liver cancer” OR “hepatic carcinoma” OR “HCC”. All retrieval processes were performed independently by two researchers.
Selection criteria
Relevant articles were screened by title and abstract after removing duplicates. Studies were eligible for inclusion if they addressed the performance evaluation of PIVKA-II and AFP for HCC diagnosis. The remaining studies were then examined in full text to confirm eligibility.
Inclusion criteria for articles were: 1) original articles reporting diagnostic accuracy of PIVKA-II and AFP for HCC in the same patients; 2) studies with sample size ≥ 30; 3) publications reporting sensitivity, specificity, and area under the ROC curve (AUC) outcomes; and 4) studies conducted in adults.
The exclusion criteria included: 1) unavailable full text electronically; 2) non-English publications; 3) comments, letters, editorials, protocols, and guidelines; and 4) studies with insufficient outcome data.
Data extraction
Two independent authors retrieved information from the eligible articles following the inclusion and exclusion criteria, and information were collected on a standardized data sheet that included: 1) study and year of publication; 2) country; 3) sample size of HCCs/controls; 4) cut-off value of PIVKA-II; 5) cut-off value of AFP; 6) etiology of HCC; 7) sensitivity of PIVKA-II/AFP; and 8) specificity of PIVKA-II/AFP.
Study quality assessment
The methodological quality of the included studies was evaluated independently, by two authors, using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool, which includes four criteria that judge bias and applicability: “patient selection”, “index test”, “reference standard”, and “flow and timing” [15]. Each is assessed in terms of risk of bias, and the first three domains were also assessed with respect to applicability. Each item is answered with “yes”, “no”, or “unclear”. The answer “yes” means low risk of bias, whereas “no” or “unclear” means the opposite. Any disagreements were resolved by inviting a third reviewer to participate in the discussion. RevMan Version 5.4 (Cochrane Collaboration, Oxford, UK) was used to visualize the quality assessment results.
Statistical analysis
Diagnostic meta-analysis of PIVKA-II and AFP was conducted on the analytical software Meta-disc 1.4 (Universidad Complutense, Barcelona, Spain) and Comprehensive Meta-Analysis version 3 (Biostat Inc., USA) in order to analyze the pooled sensitivity and specificity with 95% confidence intervals (CIs) across studies. A value of P < 0.05 was considered as the level of significance. The Cochrane Chi-squared test was used to evaluate heterogeneity among articles, with P-value < 0.05 indicating the existence of heterogeneity. To estimate the impact of heterogeneity on the meta-analysis, I2 value was calculated. I2 values ≥ 50% and P < 0.05 indicated a moderate to high degree of heterogeneity among pooled studies. A fixed-effects design was used when I2 < 50% and P > 0.05; otherwise, a random-effects model was adopted [16]. The summary receiver operating characteristic (SROC) curve, the positive likelihood ratio (PLR), the negative likelihood ratio (NLR), the diagnostic odds ratio (DOR), and the area under the curve (AUC) were also used based on the sensitivity and specificity of each study to assess the diagnostic performance. Subgroup and meta-regression analyses were performed to identify potential sources of heterogeneity according to the characteristics of the included studies. Finally, Egger’s test was conducted to evaluate publication bias. This latter was further assessed by the visual inspection of the symmetry in funnel plots.
| Results | ▴Top |
Identification of studies
The database search identified 2,879 studies to be screened, of which 1,295 abstracts were identified as potentially eligible and retrieved for full text review. Eligibility criteria were met by 53 articles, which were included to this systematic review and meta-analysis study. The PRISMA flowchart is shown in Figure 1.
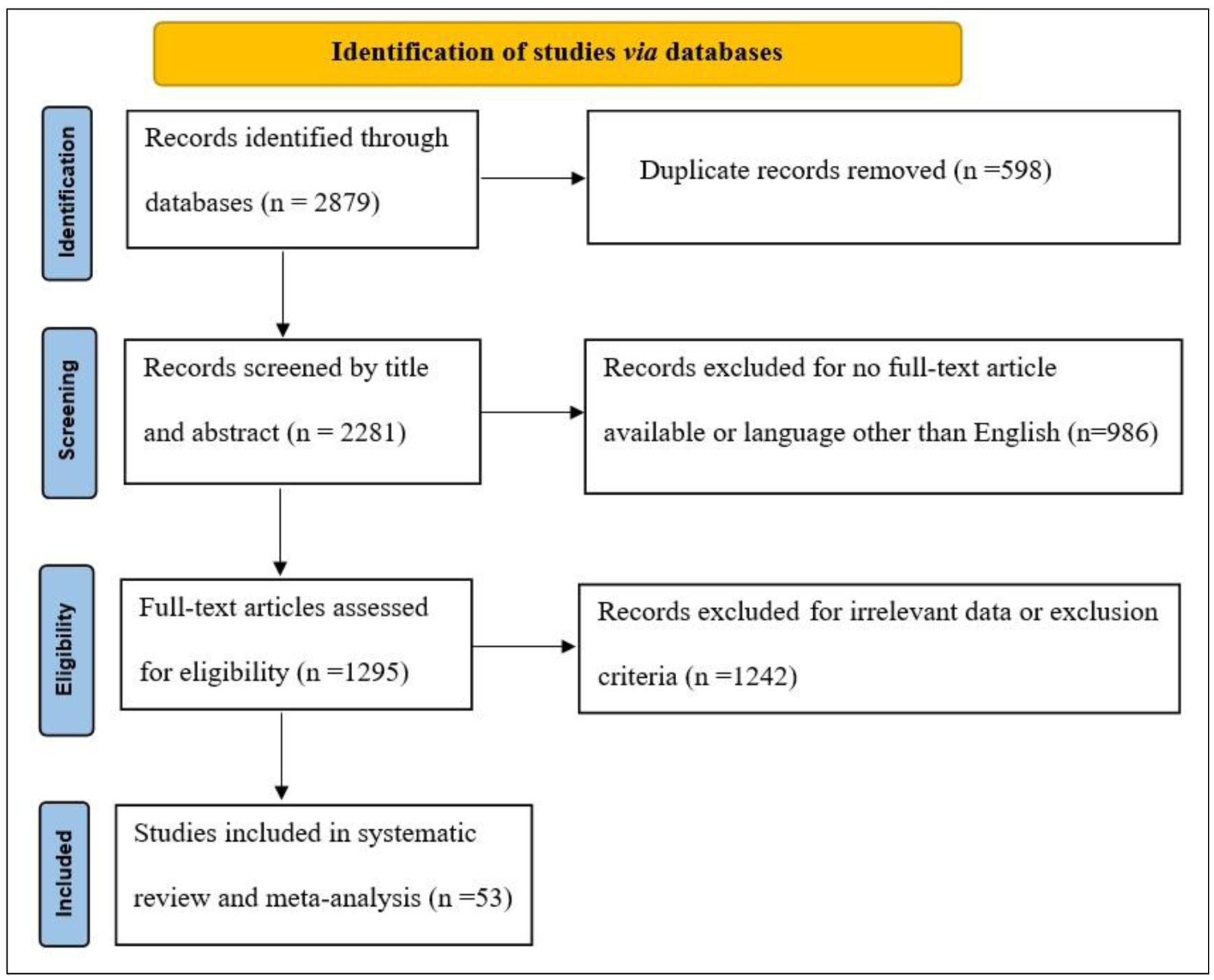 Click for large image | Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Characteristics of included studies
The included articles were published between 1989 and 2022 and distributed among 15 countries. The sample size of the included articles varied from 33 to 550 for HCC cases and from 20 to 604 for controls. The most prevalent type of control was cirrhosis (33 studies). The median/mean age of participants was > 40 years in all studies. Characteristics of included studies are summarized in Table 1 [12, 13, 17-67].
 Click to view | Table 1. Characteristics of the Studies Included in the Meta-Analysis |
Quality assessment
The quality of the 53 studies was methodologically assessed using QUADAS-2 tool. Patient selection plays such a role in conducting experiments that data used in this meta-analysis are mainly from validated groups. As a whole, the qualities of included studies are satisfying and eligible. With respect to domain patient selection, 8/53 studies were identified to have high risk of bias. However, we revealed a high-risk bias mainly concentrated on the field of index test due to presetting the threshold (20/35 studies). The domains reference standard and flow and timing were partly affected by risk of bias, with 13/53 and 12/53 studies with high risk of bias, respectively. In contrast, there were not too many concerns as for the applicability for the majority of studies included in this meta-analysis. Indeed, high applicability concerns were shown in two studies in patient selection, seven studies in index test, and two studies in reference standard, respectively. Figure 2 shows the details of the quality assessment form.
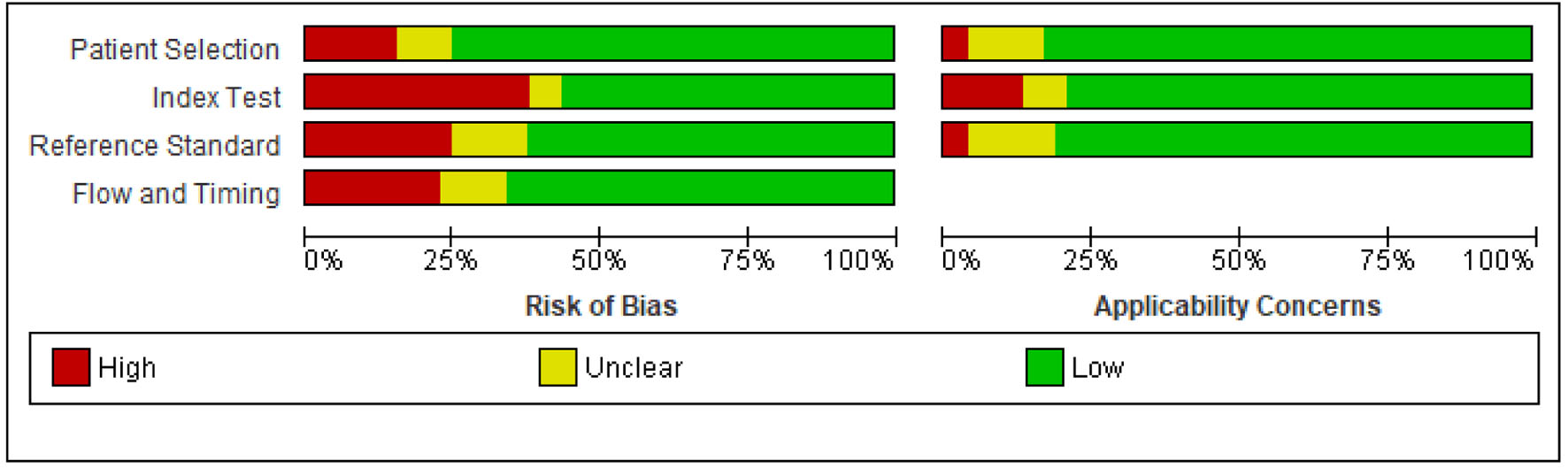 Click for large image | Figure 2. Risk of bias and applicability concerns graph: reviewing authors’ judgements about each domain presented as percentages across included studies. |
Data analysis
From forest plots of pooled data (53 studies), we found significant heterogeneity in sensitivity (Chi2 = 725.78, P = 0.0000, I2 = 92.8%) and specificity (Chi2 = 541.73, P = 0.0000, I2 = 90.4%) outcomes of PIVKA-II (Fig. 3). Similarly, a high heterogeneity was detected in sensitivity (Chi2 = 341.61, P = 0.0000, I2 = 84.8%) and specificity (Chi2 = 664.74, P = 0.0000, I2 = 92.2%) outcomes of AFP (Fig. 4). Consequently, the random-effect model was used to calculate the pooled estimates.
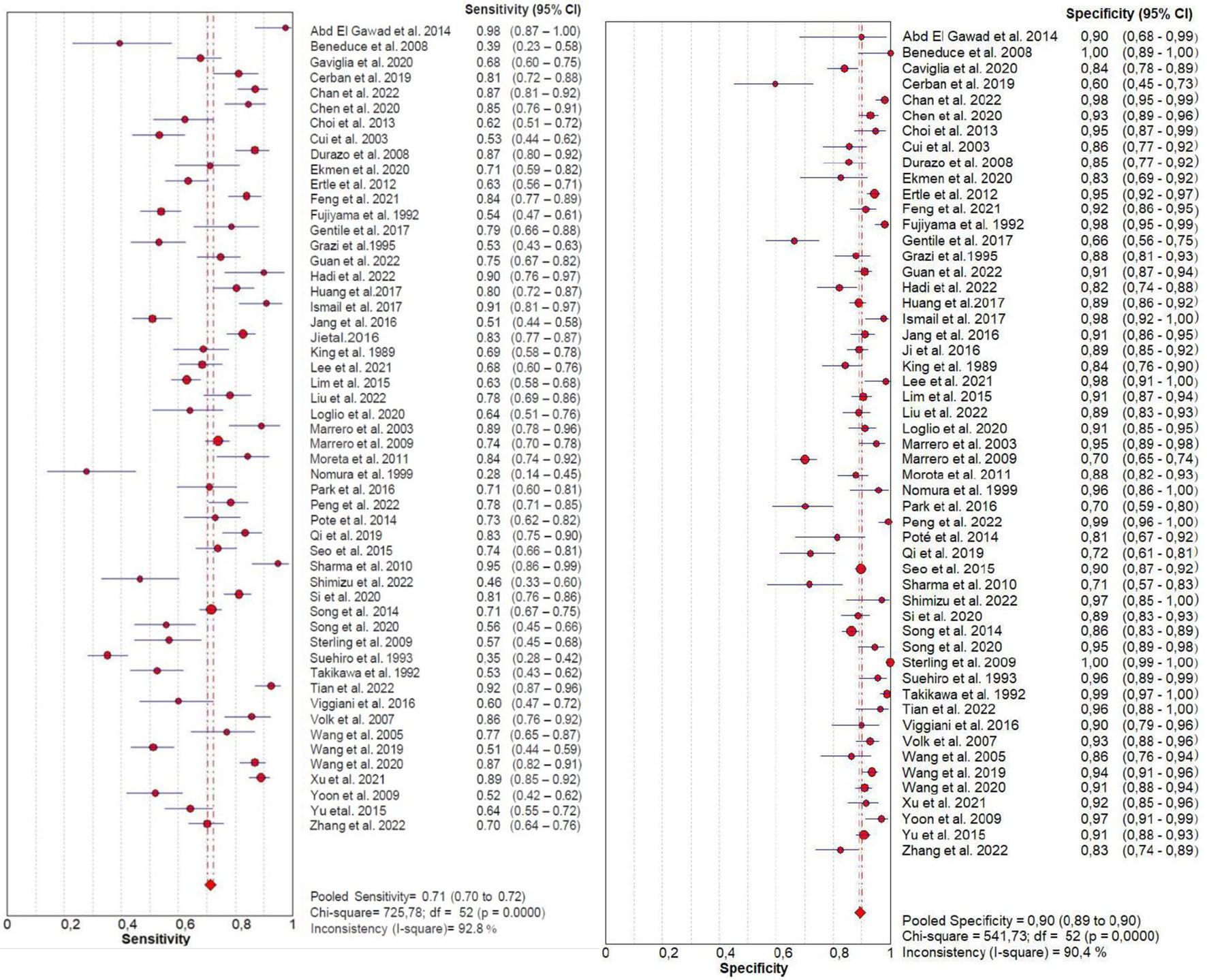 Click for large image | Figure 3. Forest plot for sensitivity and specificity of PIVKA-II. PIVKA-II: protein induced by vitamin K absence or antagonist-II. |
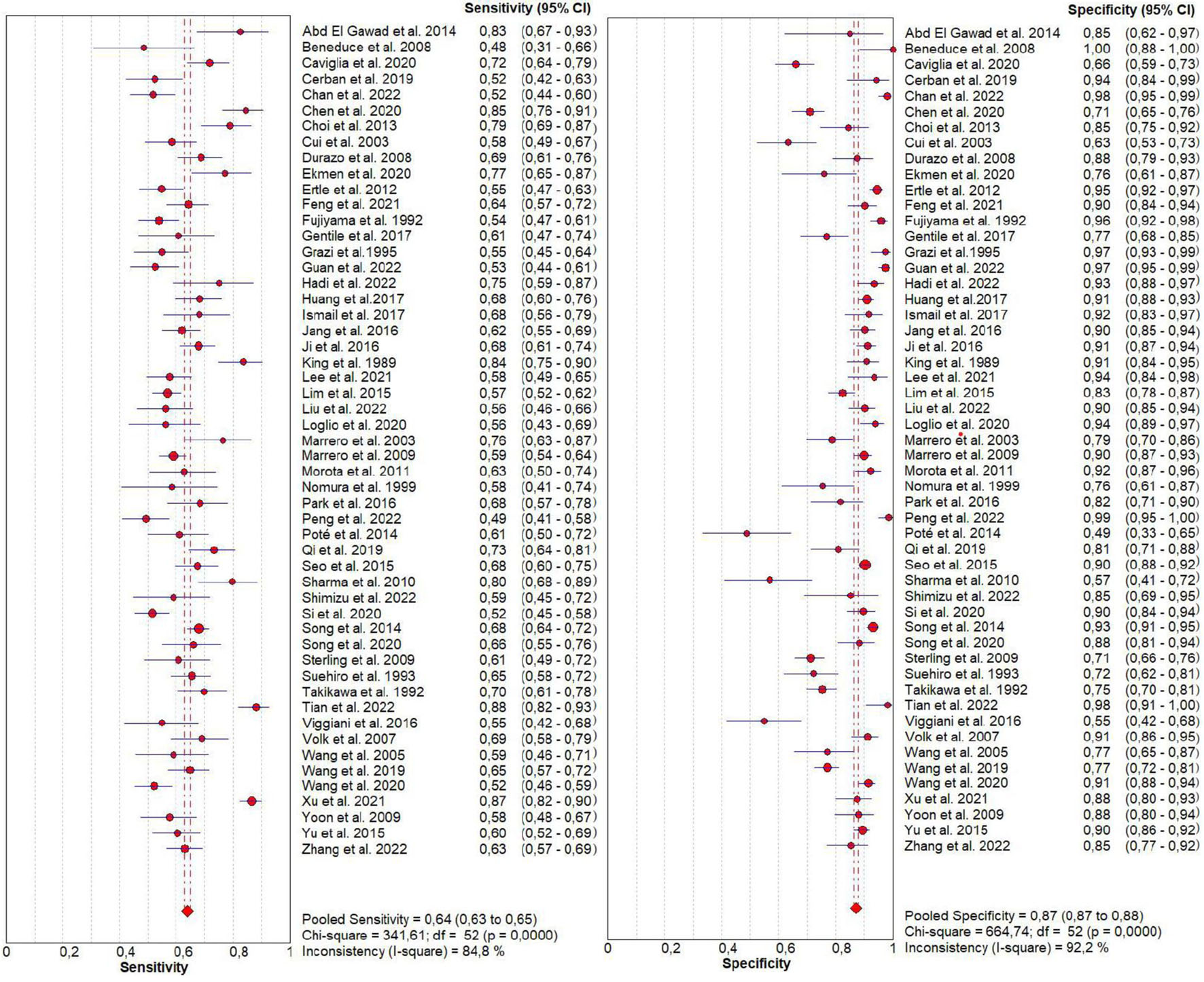 Click for large image | Figure 4. Forest plot for sensitivity and specificity of AFP. AFP: α-fetoprotein. |
The pooled sensitivity (95% CI) of PIVKA-II and AFP was 0.71 (0.70 - 0.72) and 0.64 (0.63 - 0.65), respectively, and the pooled specificity (95% CI) was 0.90 (0.89 - 0.90) and 0.87 (0.87 - 0.88), respectively. The forest plots of sensitivity and specificity derived from all the 53 studies are shown in Figure 3 and 4.
The pooled PLR (95% CI) of PIVKA-II and AFP was 7.18 (5.96 - 8.66) and 5.08 (4.20 - 6.15), and NLR (95% CI) was 0.30 (0.27 - 0.35) and 0.42 (0.39 - 0.45), respectively. The DOR (95% CI) of PIVKA-II and AFP was 27.12 (21.14 - 37.79) and 12.94 (10.35 - 16.18), respectively. Finally, the AUC of PIVKA-II and AFP was 0.89 (0.88 - 0.90) and 0.78 (0.77 - 0.79) respectively, suggesting an outstanding diagnostic accuracy of both markers (Table 2).
 Click to view | Table 2. Comparison of Diagnostic Accuracy of PIVKA-II and AFP |
Figure 5 shows the SROC curves generated from the hierarchical regression model on the overall summary of PIVKA-II and AFP. All the results showed that PIVKA-II was better than AFP in the accuracy of diagnosing HCC.
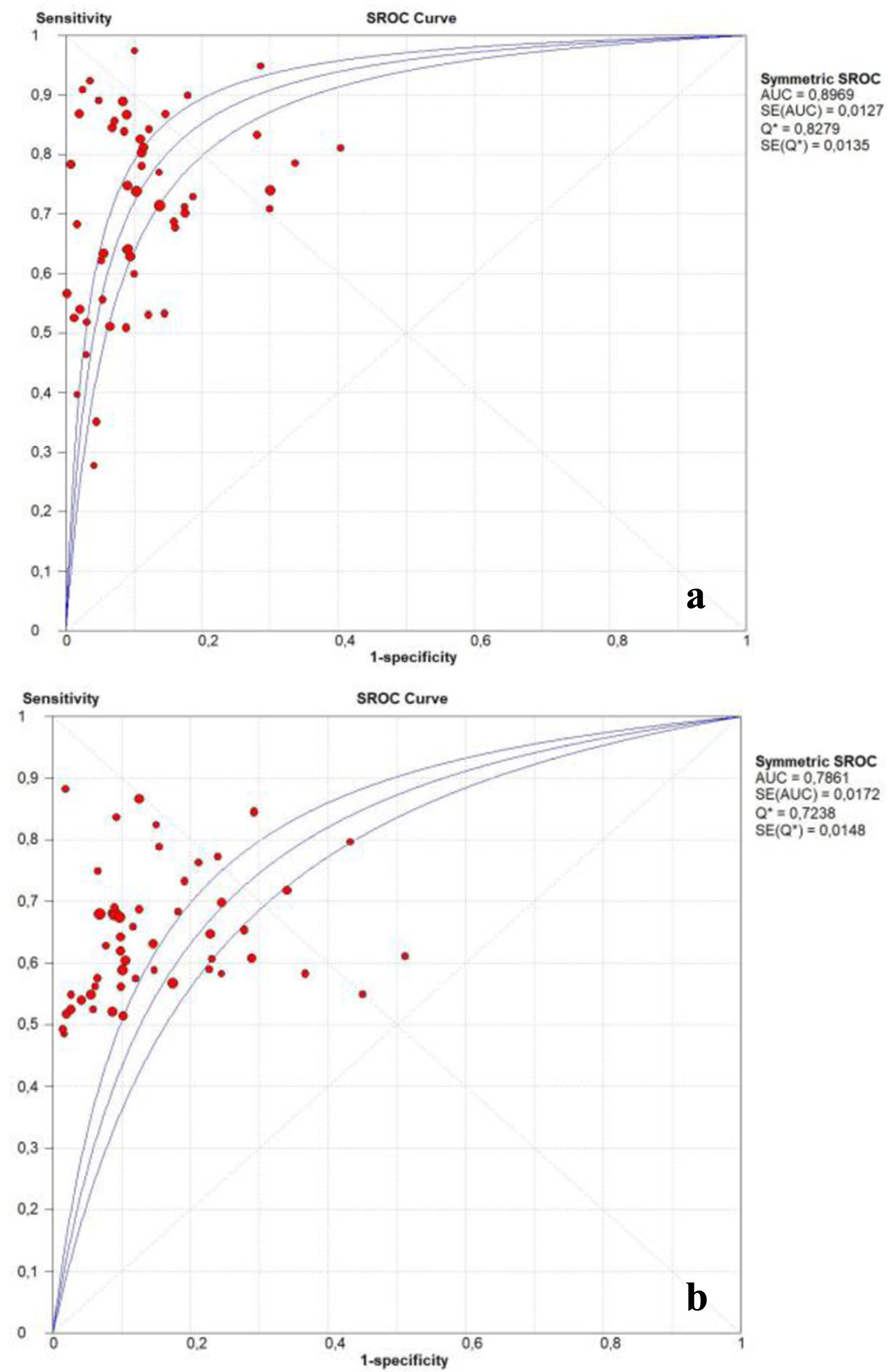 Click for large image | Figure 5. Summary receiver operating characteristic curve of the diagnostic accuracy of PIVKA-II (a) and AFP (b). AFP: α-fetoprotein; PIVKA-II: protein induced by vitamin K absence or antagonist-II. |
Investigation for heterogeneity
The forest plots demonstrated that all 53 studies were heterogeneous. As heterogeneity cannot be completely avoided in a meta-analysis, its source and level were further investigated. The threshold effect is the primary source of heterogeneity in a diagnostic test. The Moses’ model was weighted by inverse variance, and the Spearman correlation coefficient was used to assess the threshold impact. The findings revealed that the Spearman correlation coefficient for PIVKA-II was 0.284 (P = 0.039) and for AFP was 0.374 (P = 0.006). Thus, a significant threshold effect was found to cause variations in the accuracy estimates among individual studies. In order to further investigate heterogeneity based on the findings from the meta-analysis, regression analysis based on ethnicity, etiology, and sample size was also carried out. This was because it was possible that other factors may have contributed to the variation in accuracy estimates among individual studies. The results demonstrated that heterogeneity was not significantly impacted by changes in ethnicity, etiology, and sample size (P < 0.05) (Table 3).
 Click to view | Table 3. Meta-Regression Analysis of Potential Sources of Heterogeneity |
Publication bias
Funnel plot and Egger’s linear regression test were performed to evaluate the publication bias in the 53 studies. An obvious asymmetry was found when reviewing the shape of the funnel plot of the pooled DOR of PIVKA-II for diagnosis of HCC. In addition, Egger’s test revealed significant evidence of publication bias (P = 0.0004). However, no obvious asymmetry was found when reviewing the shape of the funnel plot of the pooled DOR of AFP for diagnosis of HCC. In addition, Egger’s test did not reveal any significant evidence of publication bias (P = 0.071) (Fig. 6).
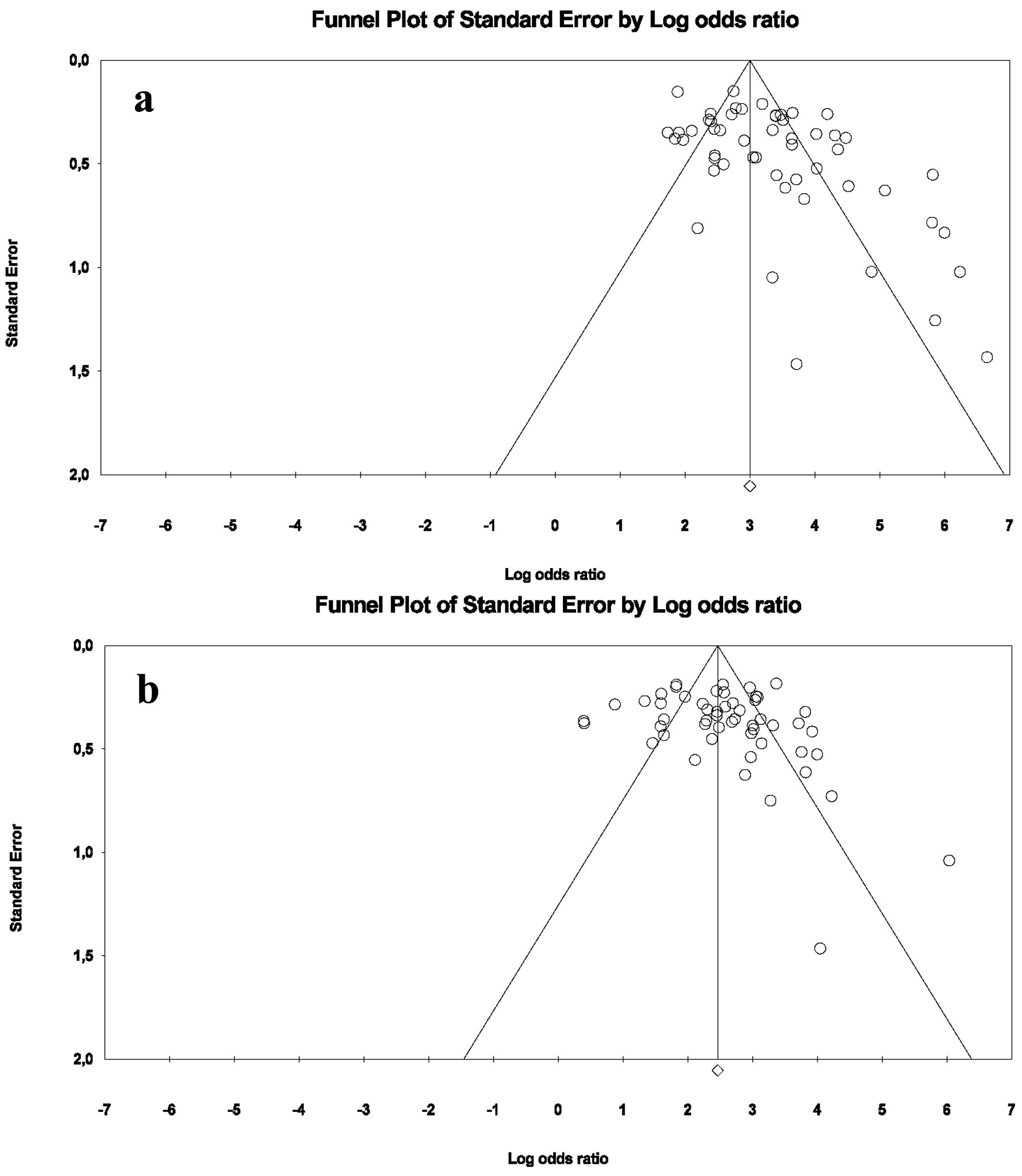 Click for large image | Figure 6. Funnel plot of the pooled DOR of PIVKA-II (a) and AFP (b) for diagnosis of HCC. AFP: α-fetoprotein; DOR: diagnostic odds ratio; HCC: hepatocellular carcinoma; PIVKA-II: protein induced by vitamin K absence or antagonist-II. |
Subgroup analysis
The pooled diagnostic accuracy of PIVKA-II or AFP for detection of HCC was analyzed in terms of ethnic group (African, European, Asian, and American patients), etiology (mixed-type HCC, HCV-related, and HBV-related) and sample size of cases (≤ 100 and > 100).
For the subgroup analysis of ethnic group, three studies were from Africa, 11 from Europe, 33 from Asia, and six from America. Higher diagnostic accuracy values of PIVKA-II were indicated in different ethnic groups compared with AFP. Interestingly, we revealed that the highest diagnostic accuracy values of PIVKA-II and AFP were detected in African patients followed by American, Asian and then European patients (Table 4).
 Click to view | Table 4. Subgroup Analysis of the Diagnostic Accuracy of PIVKA-II and AFP |
In order to further investigate the impact of HCC etiology, 41 studies dealt with HCC of mixed-etiology, four studies investigated HCV-related, and eight studies examined HBV-related. The performance of both PIVKA-II and AFP were higher when diagnosing HBV-related HCC compared to HCV-related and HCC of mixed-etiology (Table 4).
Twenty-one eligible studies reported the accuracy of PIVKA-II and AFP in diagnosing HCC with a sample size ≤ 100. The remaining studies comprised a sample size > 100. Table 4 presented the subgroup analysis of the diagnostic accuracy of PIVKA-II and AFP when considering the tumor size of HCC. The AUC of PIVKA-II was 0.903 (0.891 - 0.910) and 0.890 (0.881 - 0.896) for detection of HCC with tumor size ≤ 100 and > 100, respectively, while that of AFP was 0.773 (0.764 - 0.779) and 0.796 (0.789 - 0.802).
| Discussion | ▴Top |
In the last 10 years, the incidence rate of HCC has doubled, adding to the disease’s burden. Finding sensitive indicators for early diagnosis and monitoring of recurrence is crucial because this disease has a rapidly penetrating expansion [68]. It was shown that blood indicators for early detection of people at high risk for developing HCC present a chance to lower HCC mortality and lower medical expenses. It seems doubtful that a biochemical marker that is specifically expressed in 100% of HCCs will be discovered given the acknowledged heterogeneity of HCC. Yet, it is likely that using two or three markers in combination will boost the sensitivity of detection. To date, a number of potential biomarkers have been researched in an effort to increase the diagnosis of HCC such as PIVKA-II and AFP [20, 22, 29].
This meta-analysis showed that specificity of PIVKA-II (0.90) was almost comparable to that of AFP (0.87). However, the sensitivity and AUC of PIVKA-II were significantly higher than those of AFP, proving that PIVKA-II is more effective than AFP for detecting HCC. The AUC of PIVKA-II was considerably greater than that of AFP in detecting HCC among patients who were African, American, Asian, and European in subgroup analysis of ethnic groupings. Although AFP worked more effectively in terms of specificity in patients from Africa, PIVKA-II had higher sensitivity and AUC values. Similarly, PIVKA-II outperformed AFP in etiology subgroup analysis for diagnosing HCC with mixed, HBV-, or HCV-related etiologies. Hence, the diagnostic efficacy of PIVKA-II is deserving of future promotion in clinical practice, according to the findings of the subgroup analysis.
Three systematic review and meta-analysis studies have previously assessed the accuracy of PIVKA-II and/or AFP in HCC diagnosis. Tateishi et al reviewed 17 studies and demonstrated that PIVKA-II (AUC = 0.688) performs better in identifying HCC than AFP (AUC = 0.647) [69]. Li et al performed a systematic review to evaluate the diagnostic effectiveness of PIVKA-II and AFP in detecting HCC among 49 studies. They revealed that the AUC of PIVKA-II and AFP were 0.83 and 0.77, respectively, indicating the superiority of PIVKA- II over AFP [70]. Similarly, Xing et al showed that PIVKA-II is better than AFP in terms of the accuracy for diagnosing HCC in a meta-analysis including 31 studies [71].
These findings are consistent with the results of our study, but our study has some advantages over the above-mentioned meta-analysis in the following aspects. Firstly, we evaluated the diagnostic accuracy of PIVKA-II and AFP among HCC patients, taking studies from various nations into account (15 countries). Secondly, we used eight distinct databases for the literature search and consequently, this meta-analysis is strengthened by its broad inclusion of 53 studies and the large number of people that were examined. Thirdly, we revealed the high methodological quality of the included studies, which showed a low risk of bias.
However, this study is not without limitations. Because this meta-analysis was based on published data, it is possible that publication bias contributed to the non-significant results being less representative. Additionally, it is challenging to conduct a meta-analysis on HCC due to variations in etiology, stage of HCC, and populations. Another drawback was the use of numerous distinct cut-off values. These variations constituted a significant contributor to the inconsistencies in this meta-analysis, making it challenging to compare the findings of various research, and subsequently complicate pooled analysis. Therefore, substantial heterogeneity, which is expected in meta-analysis studies, can change how results can be interpreted [72]. In this context, we demonstrated that the primary cause of heterogeneity in this meta-analysis was the threshold effect, which arises when differences in sensitivities and specificities occur due to different cut-offs values used in different studies. However, our regression analysis failed to attribute the heterogeneity to any one of the clinical characteristics such as ethnicity, etiology, or sample size. As a result, careful consideration must be given to the present work’s findings.
The levels of PIVKA-II and AFP, which are used for diagnosing and monitoring HCC, can be influenced by various factors. Age-related genetic mutations are known to increase cancer incidence, but their impact on these markers remains unclear. Regarding sex, males have a higher HCC incidence, possibly due to greater exposure to risk factors, but it is uncertain whether this leads to differences in marker levels. Pregnant women, on the other hand, may exhibit elevated AFP levels. Smoking, a common risk factor for cancer, can indirectly affect these markers by exacerbating liver diseases through oxidative stress. AFP levels can also be elevated by other liver conditions like cirrhosis and hepatitis, as well as various benign and malignant diseases. PIVKA-II levels may increase in conditions such as vitamin K deficiency, coagulation disorders, and liver diseases.
To ensure accurate interpretation of markers like AFP and PIVKA-II, it is crucial to consider the patient’s specific condition and comorbidities. This requires a comprehensive assessment of the patient’s overall clinical status, which can be facilitated using a scale such as the Charlson Comorbidity Index. Employing such an approach allows for a more precise understanding of how AFP and PIVKA-II levels are influenced by individual patient characteristics.
However, it is important to note that published research articles still need to provide sufficient insight into the factors that may affect the levels of AFP and PIVKA-II. Further research is needed to investigate the effects of age, sex, smoking, liver conditions, vitamin K deficiency, coagulation disorders, certain types of cancer, medication use, and alcohol consumption status on the levels of AFP and PIVKA-II.
In conclusion, our analysis demonstrates that PIVKA-II is a promising biomarker for the detection and monitoring of HCC and it is more accurate than AFP. PIVKA-II has shown a higher accuracy than AFP in detecting: 1) HCC in patients from different races; 2) from both large and small sample size; and also 3) HBV-related, HCV-related or mixed-etiology HCC. To further confirm the effectiveness of PIVKA-II for HCC diagnosis, a prospective cohort study is needed.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All other authors have no conflict of interest to disclose.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: Nooraldin Merza, Zohaib Ahmed, Dushyant Singh Dahiya, Zohaib Ahmed, Mohamad Nawras, Alsadiq Al-Hillan, and Abdallah Kobeissy. Data curation: Mohamad Nawras, Mohammed Albaaj, Dushyant Singh Dahiya, Yaseen Alastal, Mona Hassan. Formal analysis: Nooraldin Merza. Project administration: Nooraldin Merza. Resources: Dushyant Singh Dahiya and Zohaib Ahmed. Software: Nooraldin Merza. Supervision: Nooraldin Merza, Abdallah Kobeissy. Visualization: Nooraldin Merza and Dushyant Singh Dahiya. The investigation, methodology, validation, writing-original draft, writing-review, and editing: all authors.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- International agency for research on cancer. The Global Cancer Observatory [Internet]. World Health Organization. 2019. Disponible sur: https://gco.iarc.fr/today/home.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917.
doi pubmed - El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
doi pubmed - Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917.
doi pubmed - Negro F. Natural history of hepatic and extrahepatic hepatitis C virus diseases and impact of interferon-free HCV therapy. Cold Spring Harb Perspect Med. 2020;10(4):a036921.
doi pubmed pmc - Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317-370.
doi pubmed pmc - Campion D, Tucci A, Ponzo P, Caviglia GP. Non-invasive biomarkers for the detection of hepatocellular carcinoma. Minerva Biotecnol [Internet]. 2019;31(1). Disponible sur: https://www.minervamedica.it/index2.php?show=R04Y2019N01A0011.
- Caviglia GP, Ribaldone DG, Abate ML, Ciancio A, Pellicano R, Smedile A, Saracco GM. Performance of protein induced by vitamin K absence or antagonist-II assessed by chemiluminescence enzyme immunoassay for hepatocellular carcinoma detection: a meta-analysis. Scand J Gastroenterol. 2018;53(6):734-740.
doi pubmed - Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34(4):570-575.
doi pubmed - Kim MN, Kim BK, Kim SU, Park JY, Ahn SH, Han KH, Kim DY. Longitudinal assessment of alpha-fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis. Scand J Gastroenterol. 2019;54(10):1283-1290.
doi pubmed - Xing H, Yan C, Cheng L, Wang N, Dai S, Yuan J, Lu W, et al. Clinical application of protein induced by vitamin K antagonist-II as a biomarker in hepatocellular carcinoma. Tumour Biol. 2016;37(12):15447-15456.
doi pubmed - Sharma B, Srinivasan R, Chawla YK, Kapil S, Saini N, Singla B, Chakraborthy A, et al. Clinical utility of prothrombin induced by vitamin K absence in the detection of hepatocellular carcinoma in Indian population. Hepatol Int. 2010;4(3):569-576.
doi pubmed pmc - Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19(3):339-346.
doi pubmed pmc - Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
doi pubmed pmc - Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536.
doi pubmed - Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111.
doi pubmed - Abd El Gawad IA, Mossallam GI, Radwan NH, Elzawahry HM, Elhifnawy NM. Comparing prothrombin induced by vitamin K absence-II (PIVKA-II) with the oncofetal proteins glypican-3, Alpha feto protein and carcinoembryonic antigen in diagnosing hepatocellular carcinoma among Egyptian patients. J Egypt Natl Canc Inst. 2014;26(2):79-85.
doi pubmed - Beneduce L, Pesce G, Gallotta A, Zampieri F, Biasiolo A, Tono N, Boscato N, et al. Tumour-specific induction of immune complexes: DCP-IgM in hepatocellular carcinoma. Eur J Clin Invest. 2008;38(8):571-577.
doi pubmed - Caviglia GP, Ciruolo M, Abate ML, Carucci P, Rolle E, Rosso C, Olivero A, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist II and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers (Basel). 2020;12(11):3218.
doi pubmed pmc - Cerban R, Ester C, Iacob S, Ghioca M, Paslaru L, Dumitru R, et al. Alpha-fetoprotein, alpha-fetoprotein-L3, protein induced by vitamin K absence, glypican 3 and its combinations for diagnosis of hepatocellular carcinoma. SGO. 2019;24(1):37.
- Chan HLY, Vogel A, Berg T, De Toni EN, Kudo M, Trojan J, Eiblmaier A, et al. Performance evaluation of the Elecsys PIVKA-II and Elecsys AFP assays for hepatocellular carcinoma diagnosis. JGH Open. 2022;6(5):292-300.
doi pubmed pmc - Chen J, Tang D, Xu C, Niu Z, Li H, Li Y, Zhang P. Evaluation of Serum GDF15, AFP, and PIVKA-II as Diagnostic Markers for HBV-Associated Hepatocellular Carcinoma. Lab Med. 2021;52(4):381-389.
doi pubmed - Cui R, He J, Zhang F, Wang B, Ding H, Shen H, Li Y, et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88(12):1878-1882.
doi pubmed pmc - Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, Busuttil RW, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23(10):1541-1548.
doi pubmed - Ekmen N, Akalin C, Akyildiz M. Predictive value of protein induced by absence of vitamin K absence or antagonist II, alpha-fetoprotein and gamma-glutamyltransferase/aspartate aminotransferase ratio for the diagnosis of hepatocellular carcinoma in transplantation candidates. Eur J Gastroenterol Hepatol. 2021;32(2):294-299.
doi pubmed - Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, Gerken G, et al. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87(2):121-131.
doi pubmed - Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21(1):401.
doi pubmed pmc - Fujiyama S, Izuno K, Yamasaki K, Sato T, Taketa K. Determination of optimum cutoff levels of plasma des-gamma-carboxy prothrombin and serum alpha-fetoprotein for the diagnosis of hepatocellular carcinoma using receiver operating characteristic curves. Tumour Biol. 1992;13(5-6):316-323.
doi pubmed - Gentile I, Buonomo AR, Scotto R, Zappulo E, Carriero C, Piccirillo M, Izzo F, et al. Diagnostic accuracy of PIVKA-II, alpha-fetoprotein and a combination of both in diagnosis of hepatocellular carcinoma in patients affected by chronic HCV infection. In Vivo. 2017;31(4):695-700.
doi pubmed pmc - Grazi GL, Mazziotti A, Legnani C, Jovine E, Miniero R, Gallucci A, Palareti G, et al. The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver Transpl Surg. 1995;1(4):249-255.
doi pubmed - Guan MC, Ouyang W, Liu SY, Sun LY, Chen WY, Tong XM, Zhu H, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist-II, lens culinaris agglutinin-reactive fraction of alpha-fetoprotein alone and in combination for early detection of hepatocellular carcinoma from nonalcoholic fatty liver disease: A multicenter analysis. Hepatobiliary Pancreat Dis Int. 2022;21(6):559-568.
doi pubmed - Hadi H, Wan Shuaib WMA, Raja Ali RA, Othman H. Utility of PIVKA-II and AFP in differentiating hepatocellular carcinoma from non-malignant high-risk patients. Medicina (Kaunas). 2022;58(8):1015.
doi pubmed pmc - Huang S, Jiang F, Wang Y, Yu Y, Ren S, Wang X, Yin P, et al. Diagnostic performance of tumor markers AFP and PIVKA-II in Chinese hepatocellular carcinoma patients. Tumour Biol. 2017;39(6):1010428317705763.
doi pubmed - Ismail MM, Morsi HK, Abdulateef NA, Noaman MK, Abou El-Ella GA. Evaluation of prothrombin induced by vitamin K absence, macrophage migration inhibitory factor and Golgi protein-73 versus alpha fetoprotein for hepatocellular carcinoma diagnosis and surveillance. Scand J Clin Lab Invest. 2017;77(3):175-183.
doi pubmed - Jang ES, Sook-Hyang J, Jin-Wook K, Yun Suk C, Philippe L, Christian B, et al. Data from: Diagnostic performance of alpha-fetoprotein, protein induced by vitamin K absence, osteopontin, Dickkopf-1 and its combinations for hepatocellular carcinoma [Internet]. Dryad. 2016:102713. Disponible sur: http://datadryad.org/stash/dataset/doi:10.5061/dryad.3n901.
- Ji J, Wang H, Li Y, Zheng L, Yin Y, Zou Z, Zhou F, et al. Diagnostic Evaluation of Des-Gamma-Carboxy Prothrombin versus alpha-Fetoprotein for Hepatitis B Virus-Related Hepatocellular Carcinoma in China: A Large-Scale, Multicentre Study. PLoS One. 2016;11(4):e0153227.
doi pubmed pmc - King MA, Kew MC, Kuyl JM, Atkinson PM. A comparison between des-gamma-carboxy prothrombin and alpha-fetoprotein as markers of hepatocellular carcinoma in southern African blacks. J Gastroenterol Hepatol. 1989;4(1):17-24.
doi pubmed - Lee Q, Yu X, Yu W. The value of PIVKA-II versus AFP for the diagnosis and detection of postoperative changes in hepatocellular carcinoma. J Interv Med. 2021;4(2):77-81.
doi pubmed pmc - Lim TS, Kim DY, Han KH, Kim HS, Shin SH, Jung KS, Kim BK, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016;51(3):344-353.
doi pubmed - Liu S, Sun L, Yao L, Zhu H, Diao Y, Wang M, Xing H, et al. Diagnostic performance of AFP, AFP-L3, or PIVKA-II for hepatitis C virus-associated hepatocellular carcinoma: a multicenter analysis. J Clin Med. 2022;11(17):5075.
doi pubmed pmc - Loglio A, Iavarone M, Facchetti F, Di Paolo D, Perbellini R, Lunghi G, Ceriotti F, et al. The combination of PIVKA-II and AFP improves the detection accuracy for HCC in HBV caucasian cirrhotics on long-term oral therapy. Liver Int. 2020;40(8):1987-1996.
doi pubmed - Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37(5):1114-1121.
doi pubmed - Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137(1):110-118.
doi pubmed pmc - Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, Chan DW, et al. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, alpha-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011;49(4):711-718.
doi pubmed - Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol. 1999;94(3):650-654.
doi pubmed - Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, Cha SW, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017;96(11):e5811.
doi pubmed pmc - Peng F, Yuan H, Zhou YF, Wu SX, Long ZY, Peng YM. Diagnostic value of combined detection via protein induced by vitamin K absence or antagonist II, alpha-fetoprotein, and D-Dimer in hepatitis B virus-related hepatocellular carcinoma. Int J Gen Med. 2022;15:5763-5773.
doi pubmed pmc - Pote N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848-854.
doi pubmed - Qi F, Zhou A, Yan L, Yuan X, Wang D, Chang R, Zhang Y, et al. The diagnostic value of PIVKA-II, AFP, AFP-L3, CEA, and their combinations in primary and metastatic hepatocellular carcinoma. J Clin Lab Anal. 2020;34(5):e23158.
doi pubmed pmc - Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3928-3935.
doi pubmed pmc - Shimizu A, Shiraki K, Ito T, Sugimoto K, Sakai T, Ohmori S, Murata K, et al. Sequential fluctuation pattern of serum des-gamma-carboxy prothrombin levels detected by high-sensitive electrochemiluminescence system as an early predictive marker for hepatocellular carcinoma in patients with cirrhosis. Int J Mol Med. 2002;9(3):245-250.
pubmed - Si YQ, Wang XQ, Fan G, Wang CY, Zheng YW, Song X, Pan CC, et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect Agent Cancer. 2020;15(1):70.
doi pubmed pmc - Song P, Feng X, Inagaki Y, Song T, Zhang K, Wang Z, Zheng S, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends. 2014;8(5):266-273.
doi pubmed - Song T, Wang L, Xin R, Zhang L, Tian Y. Evaluation of serum AFP and DCP levels in the diagnosis of early-stage HBV-related HCC under different backgrounds. J Int Med Res. 2020;48(10):300060520969087.
doi pubmed pmc - Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, Kanke F, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7(1):104-113.
doi pubmed - Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer. 1994;73(10):2464-2471.
doi pubmed - Takikawa Y, Suzuki K, Yamazaki K, Goto T, Madarame T, Miura Y, Yoshida T, et al. Plasma abnormal prothrombin (PIVKA-II): a new and reliable marker for the detection of hepatocellular carcinoma. J Gastroenterol Hepatol. 1992;7(1):1-6.
doi pubmed - Tian S, Chen Y, Zhang Y, Xu X. Clinical value of serum AFP and PIVKA-II for diagnosis, treatment and prognosis of hepatocellular carcinoma. J Clin Lab Anal. 2023;37(1):e24823.
doi pubmed pmc - Viggiani V, Palombi S, Gennarini G, D'Ettorre G, De Vito C, Angeloni A, Frati L, et al. Protein induced by vitamin K absence or antagonist-II (PIVKA-II) specifically increased in Italian hepatocellular carcinoma patients. Scand J Gastroenterol. 2016;51(10):1257-1262.
doi pubmed - Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3(2):79-87.
doi pubmed - Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, Lin TJ, et al. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol. 2005;11(39):6115-6119.
doi pubmed pmc - Wang Q, Chen Q, Zhang X, Lu XL, Du Q, Zhu T, Zhang GY, et al. Diagnostic value of gamma-glutamyltransferase/aspartate aminotransferase ratio, protein induced by vitamin K absence or antagonist II, and alpha-fetoprotein in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2019;25(36):5515-5529.
doi pubmed pmc - Wang G, Lu X, Du Q, Zhang G, Wang D, Wang Q, Guo X. Diagnostic value of the gamma-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist II in hepatitis B virus-related hepatocellular carcinoma. Sci Rep. 2020;10(1):13519.
doi pubmed pmc - Xu F, Zhang L, He W, Song D, Ji X, Shao J. The diagnostic value of serum PIVKA-II alone or in combination with AFP in Chinese hepatocellular carcinoma patients. Dis Markers. 2021;2021:8868370.
doi pubmed pmc - Yoon YJ, Han KH, Kim DY. Role of serum prothrombin induced by vitamin K absence or antagonist-II in the early detection of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol. 2009;44(7):861-866.
doi pubmed - Yu R, Ding S, Tan W, Tan S, Tan Z, Xiang S, Zhou Y, et al. Performance of protein induced by vitamin K absence or antagonist-II (PIVKA-II) for hepatocellular carcinoma screening in Chinese population. Hepat Mon. 2015;15(7):e28806.
doi pubmed pmc - Zhang SG, Huang Y. Usefulness of AFP, PIVKA-II, and their combination in diagnosing hepatocellular carcinoma based on upconversion luminescence immunochromatography. Lab Med. 2022;53(5):488-494.
doi pubmed - Perumalswami PV, Wyatt B, Bowman CA, Patel K, Mageras A, Lewis SC, Branch AD. Hepatocellular carcinoma surveillance, incidence, and tumor doubling times in patients cured of hepatitis C. Cancer Med. 2022;11(9):1995-2005.
doi pubmed pmc - Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2(1):17-30.
doi pubmed pmc - Li C, Zhang Z, Zhang P, Liu J. Diagnostic accuracy of des-gamma-carboxy prothrombin versus alpha-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol Res. 2014;44(10):E11-25.
doi pubmed - Xing H, Zheng YJ, Han J, Zhang H, Li ZL, Lau WY, Shen F, et al. Protein induced by vitamin K absence or antagonist-II versus alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: A systematic review with meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17(6):487-495.
doi pubmed - Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):e1919325.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


