| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 3, March 2022, pages 126-135
Population Status of Iodine and Its Potential Effects on Thyroid Function and Autoimmunity in Southwestern Colombia
Hernando Vargas-Uricoecheaa, e , Andry Mera-Mamianb, Beatriz Bastidas-Sanchezc, Maria Pinzon-Fernandezd, Julian Murillo-Palaciosa, Luis Ramirez-Bejaranoa
aMetabolic Diseases Study Group, Department of Internal Medicine, Universidad del Cauca, Popayan, Colombia
bEpidemiology and Statistics Research Group, Universidad CES, Medellin, Colombia
cDepartment of Social Medicine and Family Health, Universidad del Cauca, Popayan, Colombia
dDepartment of Internal Medicine, Universidad del Cauca, Popayan, Colombia
eCorresponding Author: Hernando Vargas-Uricoechea, Metabolic Diseases Study Group, Department of Internal Medicine, Universidad del Cauca, Popayan, Colombia
Manuscript submitted February 14, 2022, accepted March 2, 2022, published online March 25, 2022
Short title: Iodine, Thyroid Function, and Autoimmunity
doi: https://doi.org/10.14740/jocmr4689
| Abstract | ▴Top |
Background: This study aimed to investigate the iodine status and its potential effects on thyroid function and autoimmunity in Colombia.
Methods: This was a cross-sectional study, in population of urban and rural areas, from four geographic regions in the Department of Cauca, Colombia; the participants were 412 healthy adults, a third from rural areas. The following variables were evaluated: median urinary iodine concentration (mUIC), serum thyrotropin (TSH), clinical and ultrasonographic (US) goiter assessment, and anti-thyroid peroxidase (anti-TPO), anti-thyroglobulin (anti-Tg) and anti-TSH receptor (TRAb) concentrations.
Results: The mUIC levels were 153.9 µg/L (interquartile range (IQR): 220.06); 30% had “excessive” mUIC and a quarter had “low” mUIC. The positivity of anti-Tg and anti-TPO was higher in subjects > 60 years (P = 0.017 and P ≤ 0.001, respectively). A high prevalence of “low” mUIC was found in the “low” socioeconomic status (SES) and of “more than adequate or excessive” in the “high” SES when compared with the “medium” SES (P ≤ 0.001). The prevalence of goiter by physical examination was 41.7% and 34% by US. The highest mUIC levels were significantly more prevalent in women, in subjects with elevated TSH and in those from rural areas.
Conclusions: The population status of iodine in Colombia is U-shaped; the high prevalence of goiter, hypothyroidism, and thyroid autoimmunity can be explained by excess or deficit of iodine and by probable environmental goitrogens.
Keywords: Autoimmunity; Goiter; Iodine; Thyroid; Thyrotropin; Salt
| Introduction | ▴Top |
Iodine intake-associated disorders are serious public health issues around the world. Iodine deficiency disorders (IDDs) are pervasive throughout the planet and are related with an increased risk of perinatal mortality, mental retardation and impaired brain development, hypothyroidism, endemic goiter and poor socioeconomic development, inter alia [1, 2].
However, notwithstanding all the initiatives from various international organizations, the approximate number of people at risk of experiencing IDD is around two billion. In countries with iodine deficiency, universal salt iodization (USI) is the strategy of choice for its prevention and control. However, the universal indicators used to monitor such disorders are not always complied with, and this may account for the significantly increased prevalence of a “more than adequate” or “excessive” iodine intake in some geographical areas [3-6].
The clinical disorders associated with excessive iodine intake have been linked to thyroid autoimmunity, hypothyroidism, hyperthyroidism, goiter, thyroid nodules, among other conditions [7-9].
The primary objective of this study was to assess the iodine status and thyroid health of the adult population in the Southwest in Colombia, a geographical area classified as “free of IDD” using measures of the median urinary iodine concentration (mUIC), clinical and ultrasonographic goiter assessment, thyrotropin (TSH), free T4 (FT4), and thyroid autoantibodies: anti-thyroid peroxidase (anti-TPO), anti-thyroglobulin (anti-Tg) and anti-TSH receptor (TRAb) concentrations. Our secondary objectives were to explore the potential effects of iodine status on thyroid function and autoimmunity, as well as other probable associated factors.
| Materials and Methods | ▴Top |
Subjects
The study population comprised adults in the urban area of Popayan and three rural areas (Timbio, Bolivar and Piendamo) in the Department of Cauca, Colombia. Participants were recruited between June 30, 2018 and September 19, 2019. The sample size was based on the total number of inhabitants in the four geographical areas selected (n = 268,778), according to the data from the National Administrative Statistics Department, Colombia, 2016 [10].
Considering an estimated 10% prevalence of thyroid autoimmunity [11], a 95% confidence interval (CI) and an estimated 3% error, the sample size was estimated at 384 subjects (the total number of participants was increased by 15% to account for any potential dropouts during the study, for a total of 412 study subjects). The subject distribution was as follows: urban area, n = 266; rural area, n = 146. The sampling mode for the urban area was probabilistic (simple random) and for the rural area was non-probability based on convenience.
Ethical issues
All procedures of the present study were conducted in compliance with the Helsinki Declaration for research on human beings. This study was approved by the Ethics in Research Committee of the research vice-rector office of the Universidad del Cauca-Colombia (ID: 4656, January 25, 2018).
Experiment procedure
The inclusion criterion was adults ≥ 18 years old, and the exclusion criteria were: use of levothyroxine (or any thyroid derivative), intake of anti-thyroid agents and/or selenium or iodine containing supplements (whether constantly or intermittently) during the last 6 months; partial or total thyroidectomy and radioactive iodine therapies or procedures (over the past 24 months). The sociodemographic data (origin, socioeconomic status (SES)) were collected. A brief questionnaire was administered 48 h before to identify any family history of thyroid disease (self-reported) and salt consumption habits. In order to establish the average salt consumption per person/day, the participants received one pound of iodized salt (on the same day of the appointment), and they were told to use that salt in all their food preparations (without changing the “usual” normal amounts they used in their food) requiring salt over 48 consecutive hours; then the salt container was weighed to estimate the overall household salt consumption. To calculate the average consumption per person/day, the total amount of salt used over the 2 days was divided into the number of people usually living and eating in the household (considering at least two meals per day). The result was divided by 2, in order to estimate the average salt consumption per person/day. Anthropometric and clinical measurements were taken (weight (kg), size (cm), systolic blood pressure (SBP) (mm/Hg), diastolic blood pressure (DBP), and heart rate (beats per minute (bpm))).
Measurement
mUIC
The mUIC was measured in a casual urine sample between 7 and 9 am, using spectrophotometry (modified Sandell-Kolthoff reaction) [12]. All samples were transferred to sterile tubes, frozen for the next 24 h at -20 °C and transported for biochemical analysis. The results were classified as follows (in µg/L): < 100: low iodine intake; 100 - 199: adequate iodine intake; 200 - 299: more than adequate iodine intake; ≥ 300: excessive iodine intake [13, 14].
Thyroid function
Serum samples were obtained by venipuncture, and blood was collected in 10-mL test tubes. All samples of blood were collected and centrifuged during the routine visits. Serum samples were frozen at -80 °C and stored in a biobank in a specialized clinical laboratory (MCP, Popayan-Colombia). TSH, FT4, anti-TPO, anti-Tg and TRAb were measured using chemiluminescent immunoassay (IMMULITE® 2000 Systems Analyzers; Siemens, Munich, Germany). The coefficients of variation (CVs %) were 4.6%, 6.4%, 4.96%, 8,0% and 8.3% for TSH, FT4, anti-TPO, anti-Tg and TRAb, respectively. The results were classified as follows: TSH: 0.4 - 4.0 mIU/L (normal value); < 0.4 and > 4.0 mIU/L (abnormal value). FT4 levels were only measured in those individuals with a TSH value classified as “abnormal”. The FT4 normal range was 0.89 - 1.76 ng/dL (manufacturer cutoffs). The upper limits of normal for the assays, as denoted by the manufacturers’ reference ranges for diagnosis of thyroid autoimmunity, were used to denote a positive titer for anti-TPO (≥ 35 IU/mL), for anti-Tg (≥ 40 IU/mL) and for TRAb (≥ 0.10 IU/L).
Thyroid size
Clinically, the size of the thyroid was established according to the World Health Organization criteria as grade 0, grade I and grade II [14]. The thyroid volume was also determined with high-resolution thyroid ultrasonography (US) using a linear array 10- to 12-MHz probe (SONOACER3, SAMSUNG MEDISON). Longitudinal and transverse scans were performed to measure depth, width, and length of each lobe. Thyroid lobe volume was calculated as 0.479 × depth × width × length (cm). Thyroid volume was calculated as the sum of the volumes of both lobes, excluding the isthmus. Goiter was defined as a volume ≥ 12.5 mL in females and ≥ 15 mL in males [15, 16].
Statistical analysis
The qualitative variables were analyzed using frequencies and percentages. The quantitative variables were analyzed or the normality of the quantitative variables was evaluated using the Shapiro-Francia normality test to establish the use of parametrical statistics (median and standard deviation (SD)) or non-parametric statistics (median and interquartile range (IQR)). To assess the existing relationship between the independent variables (mUIC, age, gender, SES, body mass index (BMI)) and the dependent variables (goiter (yes/no) and thyroid function), a negative binomial regression and a logistical regression were used. The prevalence ratios (PRs) were estimated and a bivariate analysis using X2 was conducted to identify the variables for inclusion in the regressions; subsequently, the simple regressions were analyzed to calculate the PR with their corresponding 95% CI. Finally, a multivariate analysis was conducted to identify the adjusted PR and analysis of variance (ANOVA) and Tukey test were used to determine the significant differences between the groups of mUIC and the relevant variables. The data were captured in Excel and were then transferred to the R program version 4.0.2 using the Nortest Library and SPSS version 25.
| Results | ▴Top |
Baseline characteristics of the participants and salt consumption habits
A total of 442 individuals were assessed, of which 21 from the urban area and nine from the rural area were excluded; finally, 412 individuals were admitted to the study (Fig. 1).
 Click for large image | Figure 1. Flowchart of the participants included in the trial. mUIC: median urinary iodine concentration. |
Most of the subjects were females and the average age was 42.5 years (SD: 14.62); the majority came from the urban area and were from a low SES. The average size was 158 cm (SD: 8.164), with a body weight of 67.5 kg (SD: 12.90) and a BMI of 26.7 kg/m2 (SD: 4.39). The mean SBP and DBP were 119.6 (SD: 12.20) and 72.7 (SD: 7.43) mm Hg, respectively, and the mean heart rate was 71.4 bpm (SD: 6.30). Over one-third of the participants reported a family history (first-degree relative) of thyroid dysfunction, abnormal thyroid size, or a diagnosis of thyroid cancer (Table 1).
 Click to view | Table 1. Sociodemographic and Anthropomorphic Characteristics, and Family History of Thyroid Disorders |
Of the subjects, 99.2% claimed using salt for human consumption and 94% said they used iodized salt; the average consumption of salt/person/day was 13.2 g (SD: 4.21), distributed as follows: 14.3 g in the urban area and 12.1 g in the rural area; based on the SES, the distribution was as follows: low SES, 13.9 g; middle SES, 14.2 g; and high SES: 14.8 g. No differences were found in the intake of salt based on origin or SES (P = 0.26).
Differences between the presence of thyroid autoimmunity and distribution according to age and origin
The distribution of thyroid autoantibodies was as follows: 22/412 (5.3%) of the participants were TRAb-positive; 10% were anti-Tg-positive; 18.2% were anti-TPO-positive (31.3% of the participants had at least one of the three positive autoantibodies). The distribution of thyroid autoantibodies according to age (< 60 or ≥ 60 years) was as follows: for TRAb, of the 360 subjects < 60 years, 20 of them (5.5%) were TRAb-positive; in contrast, subjects ≥ 60 years, 2/52 were TRAb-positive (3.84%) (X2: 0.263; odds ratio (OR): 0.680; 95% CI: 0.154 - 2.998 (P = 0.608)). Moreover, in terms of anti-Tg positivity, it was documented in 31/360 (8.61%) and in 10/52 (19.23%) of the subjects < 60 and ≥ 60 years, respectively (X2: 5.718; OR: 2.527; 95% CI: 1.156 - 5.522 (P = 0.017)). Anti-TPO positivity was reported in 57/360 (15.83%) among the general population, and in 57/360 (15.83%) and in 18/52 (34.61%) in < 60 and in ≥ 60 years, respectively (X2: 10.765; OR: 2.814; 95% CI: 1.488 - 5.324 (P ≤ 0.001)). Similarly, the positivity of thyroid antibodies based on origin (urban vs. rural) for TRAb, was 8/146 (5.47%) and 14/266 (5.26%) (X2: 0.009; OR: 0.958; 95% CI: 0.392 - 2.341 (P = 0.926)); for anti-Tg, was 13/146 (8.9%) and 28/266 (10.52%) (X2: 0.277; OR: 1.204; 95% CI: 0.603 - 2.40 (P = 0.599)). Finally, with regards to anti-TPO, positivity was present in 22/146 (15.1%) and 53/266 (19.9%) (X2: 1.493; OR: 1.402; 95% CI: 0.814 - 2.417 (P = 0.222)).
Differences in terms of mUIC and distribution according age, SES and thyroid autoantibodies
Table 2 shows the distribution of mUIC based on gender, SES and origin. The mUIC in the population was 153.9 µg/L (IQR: 220.06); 100 subjects (24.31%) had low mUIC, 176 (42.7%) had adequate mUIC, 13 (3.2%) more than adequate mUIC and 123 (29.9%) had values ≥ 300 µg/L. mUIC in females was 149 µg/L (IQR: 216.68) and in males 182 µg/L (IQR: 268.7); among individuals who claimed not to use iodized salt, mUIC was 149 µg/L and among those using iodized salt was 153 µg/L. The distribution according to age was as follows: 18 - 39.9 years (148.7 µg/L); 40 - 59.9 years (147.8 µg/L); ≥ 60 years (289 µg/L). The mUIC among the low SES was 144 µg/L (IQR: 122.5); among the middle SES was 184.7 µg/L (IQR: 255.8); and in the high SES was 311.3 µg/L (IQR: 277). Significant differences were identified between the presence of “low” mUIC and a “low” SES and a “more than adequate or excessive” mUIC and the “high” SES in contrast with the middle SES (X2: 38.266; P ≤ 0.001). The bivariate analysis (Log-binomial regression) also showed a significant difference in terms of origin and level of mUIC, with a higher prevalence of excessive mUIC among the rural population (PR: 3.11; 95% CI: 1.88 - 5.34; P ≤ 0.001). Moreover, the distribution of mUIC based on BMI was as follows: low BMI, 123.3 µg/L (IQR: 87.94); normal, 169.2 µg/L (IQR: 239.2); overweight, 154.6 µg/L (IQR: 284.1); obese, 132.7 µg/L (IQR: 105). There were no significant differences in terms of mUIC and the presence of thyroid autoantibodies (Fig. 2).
 Click to view | Table 2. Distribution of mUIC According to Gender, SES and Origin |
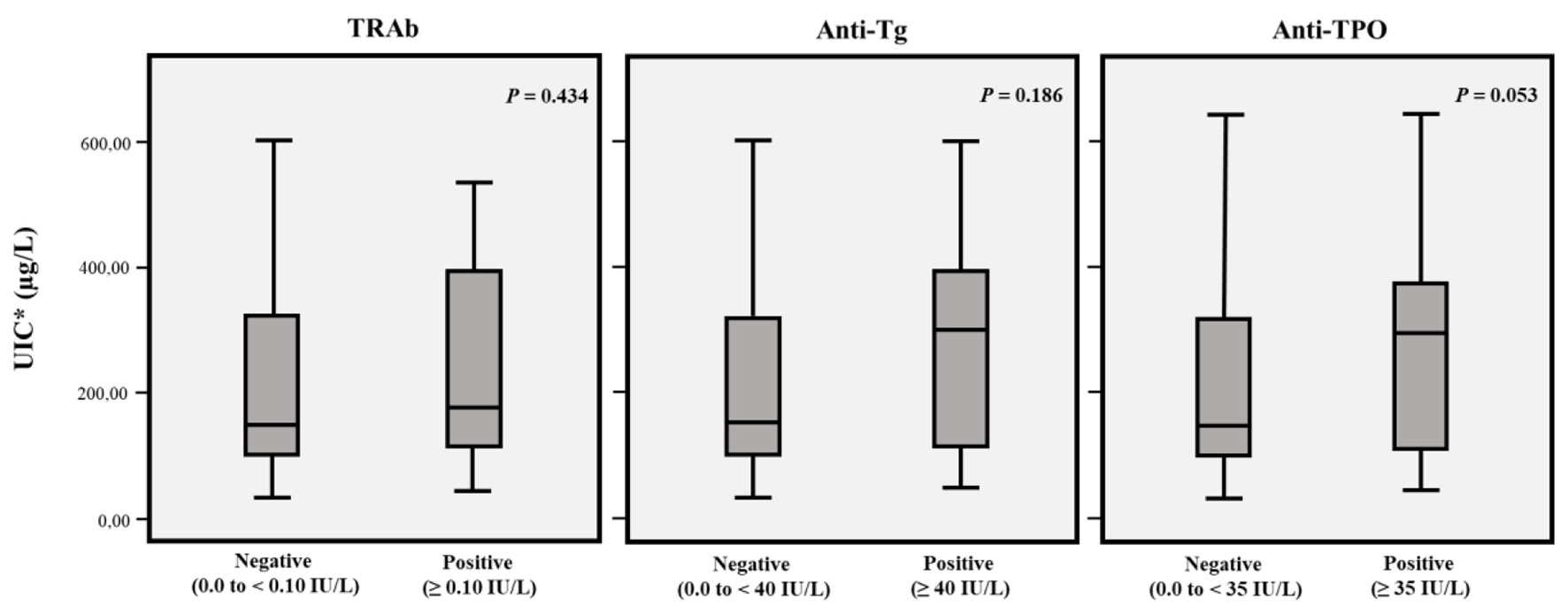 Click for large image | Figure 2. mUIC according to negative or positive TRAb, anti-Tg and anti-TPO. *mUIC (µg/L) in TRAb negative or positive subjects was 152.8 (IQR: 219.7) and 178.7 (IQR: 287.4), respectively. In anti-Tg negative or positive subjects mUIC (µg/L) was 150.5 (IQR: 219) and 301 (IQR: 289.2), respectively. In anti-TPO negative or positive subjects mUIC (µg/L) was 152 (IQR: 221.4) and 176 (IQR: 236.8), respectively. mUIC: median urinary iodine concentration; TRAb: anti-TSH receptor; anti-Tg: anti-thyroglobulin; anti-TPO: anti-thyroid peroxidase. |
Differences between mUIC and thyroid function
The mUIC among TSH < 0.4 mIU/L individuals was 381.8 µg/L (but just 1.45% of the subjects had suppressed TSH; hence, no analyses were conducted in this particular subgroup); 144 µg/L for those with a TSH between 0.4 and 4.0 mIU/L and, 191.9 µg/L among the participants with a TSH > 4.0 mIU/L. Of the participants, 324 (78.6%) had a normal range TSH; 84 (20.4%) had high values (70/84 were FT4 normal (83.3%) and 14/84 (16.6%) had low FT4 levels); finally, four exhibited a suppressed TSH (all of normal FT4) (Table 3).
 Click to view | Table 3. Comparison of the Clinical Characteristics of Adults With Various mUIC |
Differences between thyroid function and age, gender, BMI, SES and mUIC
The multiple regression model showed a significant relationship between the presence of hypothyroidism and age ≥ 60 years in females and a low SES. The frequency of hypothyroidism was higher among individuals with excessive iodine intake. In the simple regression results (and ANOVA) for variables such as age, gender, SES and mUIC, significant differences were identified in at least one of category. No differences were found between the presence of hypothyroidism and BMI (X2 = 2.5428; P = 0.2804), neither between participants with “low” mUIC and variables such as age, origin and SES (Tables 3 and 4).
 Click to view | Table 4. Adjusted PR for Hypothyroidisma |
Differences between the prevalence of goiter established with a physical examination vs. US
The physical examination identified 172 subjects with goiter (41.7%), of which 39% were grade I and 2.7% were grade II. With US, 140 (34%) of the individuals were identified with goiter. Among the individuals with a normal thyroid volume according to US (n = 272), 46 (26.7%) had clinical goiter; among those with goiter according to the US examination, clinical goiter was identified in 126/172 (73.3%) (kappa index = 0.191; standard error (SE): 0.013).
Differences between the presence of goiter, thyroid autoimmunity and SES and between excessive mUIC, gender, TSH, origin and SES
With regards to goiter distribution (per clinic) according to mUIC, of the total number of subjects with goiter (n = 172), 45 (26.2%) individuals with mUIC < 100 µg/L had goiter and for levels of mUIC of 100 - 199 µg/L, 200 - 299 µg/L and > 300 µg/L, the proportion of goiter was 46.5%, 3.5%, and 23.8%, respectively. Of the total number of participants with goiter, 38/172 (22.1%) were anti-TPO-positive, as compared to those without goiter: 37/240 (15.4%) (X2: 2.999; OR: 1.556; 95% CI: 0.941 - 2.572 (P = 0.083)). No differences were found between anti-Tg and TRAb positivity and the presence or absence of goiter (X2: 0.002; OR: 1.013; 95% CI: 0.526 - 1.950 (P = 0.969) and X2: 1.565; OR: 0.580; 95% CI: 0.245 - 1.374 (P = 0.211)), respectively. With regards to the middle SES participants, the prevalence of goiter was 40% less than in the high SES (X2 = 9.2717; PR: 0.60; 95% CI: 0.39 - 0.91 (P = 0.02)) (Table 5). There were no differences between the presence of goiter and the levels of mUIC (X2 = 5.1117; P = 0.08), age (X2 = 5.4139; P = 0.07), and gender (X2 = 0.69137; P = 0.41). However, for a level of mUIC ≥ 300 µg/L, some differences were identified with regards to variables such as: gender ((higher proportion of females with excess mUIC as compared to males) (PR: 1.916; 95% CI: 1.089 - 3.370, P = 0.011)); and elevated TSH (PR: 1.726; 95% CI: 1.015 - 2.935, P = 0.010). No differences were identified between a level of mUIC < 300 µg/L and gender (X2: 7.239; P = 0.065).
 Click to view | Table 5. Adjusted PR for Goiter and Excessive mUICa |
| Discussion | ▴Top |
This study evidences a “U-shaped” iodine intake distribution in the southwestern region of Colombia, which is classified as an IDD-free area. One-third of the individuals have a high iodine intake (or at least more than adequate), while one-fourth of the population exhibits a low intake, with a higher prevalence of iodine deficiency in the lower SES and a higher prevalence of excessive iodine intake in the high SES; this is also the case for the rural population. These results are consistent with other national studies. For instance, prior to the introduction of the USI program in 1947 in Colombia, the iodine status in the population was established based on the rate of goiter, which for that time was 53% (in some regions the rate was > 80%). Following the implementation of the USI program, the rate of goiter in Colombia dropped to < 2% in 1965 [8]. However, more recently, the national survey on Nutritional Status (Colombia, 2015) found that 75% of school-age children (5 - 12 years) and 70% of women in child-bearing age (13 - 49 years) had excessive iodine levels and 4.4% and 4.9% presented iodine deficiency, respectively. Iodine deficiency was more prevalent in the rural area and iodine excess was more prevalent in the urban area [9].
Additionally, the fact that there were no differences in terms of origin and SES with regards to the average consumption of salt per day, indicates that probably the findings associated with iodine excess or deficiency may be due to factors such as the high intake of food products with high iodine content or some cooking habits, such as the use of concentrated chicken, meat, or fish stock (high in iodized salt) or the non-reported or unknown use of iodine-containing vitamin supplements (for the high SES and for individuals in the rural areas), or maybe wider access to low iodine-content salt (because of cultural beliefs or preferences) or simply because people in certain populations think that the salt they use is iodized when in fact it is not (the low SES groups). Regardless of the situation, evidently the public health programs to monitor and control iodine levels in the population are not being implemented [17, 18].
Similarly, the average salt consumption in this population (13.23 g/day) is far above the international daily recommended level (< 5 g/day). We had previously shown that in school-age children (< 12 years) the average salt consumption was 18.13 g/day [19, 20]. This indicates - at least in part - the lack of educational and awareness programs about the consequences of excessive salt intake early in life, not just because of the risk of thyroid dysfunction, but also because of cardiovascular outcomes such as the risk of hypertension, inter alia.
This study found a significant relationship between anti-TPO and anti-Tg positivity in individuals > 60 years old. This finding has also been documented in other trials showing the relationship between age and a higher frequency of positive thyroid antibodies [21-23]. However, the association between a higher frequency of positive thyroid antibodies and older individuals has not been reproducible in other studies; hence, longitudinal studies should be conducted to elucidate this matter. The higher frequency of thyroid autoimmunity in our population could be influenced by environmental factors, nutritional habits, or vitamin-D, selenium, and iron deficiency and smoking, among other factors [24, 25].
Furthermore, over one-third of the population studied reported a first-degree relative with some sort of thyroid disorder, which is consistent with other previous studies that reported a “cluster” distribution of the autoimmune thyroid disease, with 40-50% of the affected individuals reporting a close relative with a thyroid condition [26].
Moreover, the high frequency of hypothyroidism in our population is highly noticeable (> 20%), since it has been established that the prevalence of primary hypothyroidism among the general population ranges between 0-3% and 3-7% in the US, and between 0-2% and 5.3% in Europe (depending on the definition used) [27].
A meta-analysis of seven trials assessing the prevalence of “undiagnosed” hypothyroidism in nine European countries (including primary and subclinical hypothyroidism) reported a 5% prevalence, with an estimated incidence of 226.2 (222.26 - 230.17) per 100,000 per year [28].
These data are in contrast with the frequency of hypothyroidism in the Japanese population, where the frequency of both primary and subclinical hypothyroidism is around 8%, or in India, where the prevalence of subclinical hypothyroidism has been estimated at 19.3% [29, 30].
Other studies also show an increased frequency of hypothyroidism with ageing; for instance, in Brazil and in Australia, the prevalence of hypothyroidism among the elderly population was 5.7% and 0.7%, respectively [31, 32].
However, hypothyroidism is more frequent in females than in males (with an incidence peak between 30 and 50 years). For instance, in the United States of America, hypothyroidism affects around 4% of the women between 18 and 24 years old and 21% of women over 74 years old [33, 34].
The lifetime risk of developing primary hypothyroidism has been found at 4.1% for females and 1.3% for males. The differences found in regards to the frequency of hypothyroidism in our population with regards to other populations may be due to various reasons; for instance, the “U-shape” population distribution for iodine intake (one end deficiency and one end excess) is certainly associated with an increased risk of hypothyroidism; and the high frequency of thyroid autoimmunity, both could account for the increased prevalence of hypothyroidism in our population. However, it should be kept in mind that the comparison of epidemiological studies assessing the frequency of thyroid dysfunction could be complex, since the result may be influenced by factors such as the definition or the determination of a universal cut point for the TSH value (that adequately classifies the patient with thyroid dysfunction) or the selection criterion of the population assessed, or by age, gender, and environmental or genetic factors including race, inter alia [35].
The high prevalence of thyroid autoimmunity in this population (> 30%) is also striking, and this may be due - at least in part - to the fact that in geographical regions where USI programs are introduced, the frequency of anti-Tg is increased, probably as a result of an excessive iodine intake (or due to the introduction of USI programs in deficient areas) which could potentially lead to a higher antigenicity of Tg, and hence increased stimulus for the secretion of anti-Tg antibodies and a higher frequency of thyroid autoimmunity [36, 37].
The prevalence of goiter was also associated with excessive or insufficient iodine levels; there was for instance a significant association between elevated mUIC (≥ 300 µg/L) and the prevalence of goiter (specifically in women, in individuals with elevated TSH and in individuals from the rural areas); in contrast, there was an inverse relationship between individuals from a middle SES and the presence of goiter. The explanation for this situation could be that the higher the iodine intake, the higher the frequency of goiter (and higher TSH levels). Another argument is that overall, women take larger amounts of iodine from food (as compared to men). However, the prevalence of goiter in the population could be associated to excessive or insufficient consumption of iodine, to genetic and/or hereditary factors, to anthropometric and/or environmental factors, or to the presence of goitrogens, inter alia. A significant relationship was found between excessive mUIC and the prevalence of goiter, but not for all ranges of mUIC; therefore, the high prevalence of documented goiter in our population shall also take into consideration factors other than iodine intake (for instance, the presence of goitrogens and/or endocrine disruptors) [38].
This study failed to identify a good concordance between the physical examination and thyroid US; the prevalence of goiter determined through inspection and palpation was higher than using ultrasound. Nonetheless, the prevalence of goiter using US was high (34%), and suggests that the identification of goiter through inspection and palpation may be overestimating the actual prevalence of goiter. One should then keep in mind that the WHO classification is more qualitative than quantitative and could in part account for the higher prevalence of goiter identified through physical examination (such classification may overestimate the true prevalence of goiter in the population). Moreover, in Colombia there is a lack of population-based studies establishing the thyroid volume ranges based on variables such as age, gender, body weight, size, etc.; therefore, the results of this study may only be extrapolated to the established definition of goiter (12.5 mL in women and 15 mL in men). The higher performance and diagnostic procedure of US indicates that this should be the method of choice to determine and classify goiter in population studies [39, 40].
This study has some limitations; for instance, we assessed the self-reported family history of thyroid disease and habits associated with salt consumption via a questionnaire, which may have a recall bias. Additionally, the coverage of households with access to properly iodized salt in the diet may only provide limited information about the total salt intake in the diet and hence other significant sources of salt in the diet would not be represented in this study. Neither did we assess other factors that potentially and independently impact the thyroid function and the presence of autoimmunity; i.e., the presence of smoking, deficiency of other micronutrients, the presence of goitrogens and/or endocrine disruptors. The probable daily variations in individual iodine intake and mUIC should also be taken into account [14]. It should also be noted that mUIC can be influenced by kidney function and other conditions such as dehydration; for example, on an “individual” basis, a 24-h urine sample is considered necessary to assess iodine intake (since the level is more constant in iodine-deficient populations than in those with adequate intake); whereas, on a “population” basis, mUIC (in a randomly selected random sample of a random urine sample) has been shown to provide useful information on average iodine intake or iodine status community. This study, having a “population” base, complies with said recommendation [2, 14].
Conclusions
A considerable number of individuals in our population fail to achieve normal iodine levels, which could explain the high prevalence of goiter, hypothyroidism, and thyroid autoimmunity. It is imperative to ensure that monitoring and follow-up programs for iodine status of the population are developed and implemented. This study may encourage research on monitoring and follow-up of the impact of iodine on other health outcomes. Further studies are needed to validate our findings and to assess the deficiency or excess of iodine in other regions and in specific Colombian populations.
Acknowledgments
The authors thank all the participants, the hospitals, clinics, community action boards, and community leaders who made this study possible. We also give thanks to the specialized clinical laboratory Martha Cecilia Perdomo. Likewise, we show our deep gratitude to the Colombian association of endocrinology and to the Universidad del Cauca for the logistical and infrastructure support.
Financial Disclosure
This study received funding from the Colombian Association of Endocrinology, Diabetes and Metabolism and from the Vice-rector for Research of the Universidad del Cauca.
Conflict of Interest
The authors have nothing to disclose.
Informed Consent
Consent was obtained from each subject after full explanation of the purpose and nature of all the procedures used.
Author Contributions
HVU, BBS, MPF, JMP and LRB designed the study. HVU, AMM and BBS performed the statistical analyses and created the figures. HVU, AMM and BBS drafted the manuscript. HVU, AMM and BBS interpreted the data and edited the manuscript. All authors have given final approval of the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
Anti-Tg: anti-thyroglobulin; anti-TPO: anti-thyroid peroxidase; BMI: body mass index; bpm: beats per minute; CI: confidence interval; CVs: coefficients of variation; DBP: diastolic blood pressure; FT4: free T4; IDDs: iodine deficiency disorders; IQR: interquartile range; mUIC: median urinary iodine concentration; PR: prevalence ratio; SBP: systolic blood pressure; SD: standard deviation; SES: socioeconomic status; TRAb: anti-TSH receptor; TSH: thyrotropin; USI: universal salt iodization
| References | ▴Top |
- Vanderpas JB, Moreno-Reyes R. Historical aspects of iodine deficiency control. Minerva Med. 2017;108(2):124-135.
doi pubmed - Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286-295.
doi - Vanderpump MP. Epidemiology of iodine deficiency. Minerva Med. 2017;108(2):116-123.
doi pubmed - Li M, Eastman CJ. The changing epidemiology of iodine deficiency. Nat Rev Endocrinol. 2012;8(7):434-440.
doi pubmed - Andersson M, Takkouche B, Egli I, Allen HE, de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83(7):518-525.
- Zhao W, Han C, Shi X, Xiong C, Sun J, Shan Z, Teng W. Prevalence of goiter and thyroid nodules before and after implementation of the universal salt iodization program in mainland China from 1985 to 2014: a systematic review and meta-analysis. PLoS One. 2014;9(10):e109549.
doi pubmed - Teti C, Panciroli M, Nazzari E, Pesce G, Mariotti S, Olivieri A, Bagnasco M. Iodoprophylaxis and thyroid autoimmunity: an update. Immunol Res. 2021;69(2):129-138.
doi pubmed - Ministerio de Salud, Instituto Nacional de Salud, Instituto Colombiano de Bienestar Familiar, Sociedad Colombiana de Endocrinologia, UNICEF-OPS/ OMS, Colciencias. Prevalencia de los Desordenes por Deficiencia de Yodo e Ingestion Promedio de Sal. Colombia, 1994-1998. Santa Fe de Bogota, D.C., primera edicion; noviembre de 2001. ISBN 958-13-0129-1.
- https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/PSP/presentacion-lanzamiento-ensin-2015.pdf. Accessed March 05, 2021.
- https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/proyecciones-de-poblacion. Accessed December 2017.
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252-265.
doi pubmed - Pino S, Fang SL, Braverman LE. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem. 1996;42(2):239-243.
doi pubmed - EFSA. Scientific opinion on dietary reference values for iodine. EFSA J. 2014;12(5):3660.
doi - World Health Organization-WHO. International Council for Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. A guide programmed managers, 2nd edition. Geneva, Switzerland, WHO, Department of Nutrition for Health and Development; 2001. (WHO/ NHD/01.1).
- Viduetsky A, Herrejon CL. Sonographic evaluation of thyroid size: a review of important measurement parameters. J Diagn Med Sonogr. 2019;35(3):206-210.
doi - Dighe M, Barr R, Bojunga J, Cantisani V, Chammas MC, Cosgrove D, Cui XW, et al. Thyroid ultrasound: state of the Art Part 1 - thyroid ultrasound reporting and diffuse thyroid diseases. Med Ultrason. 2017;19(1):79-93.
doi pubmed - Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70(10):553-570.
doi pubmed - von Oettingen JE, Brathwaite TD, Carpenter C, Bonnell R, He X, Braverman LE, Pearce EN, et al. Population survey of iodine deficiency and environmental disruptors of thyroid function in young children in Haiti. J Clin Endocrinol Metab. 2017;102(2):644-651.
doi pubmed - Vargas-Uricoechea H, Bastidas-Sanchez B, Perdomo-Cabrera M, Vargas-Sierra H. Estado Nutricional del Yodo. Implicacion en la positividad de anticuerpos antitiroideos y posible autoinmunidad tiroidea en una poblacion escolar declarada "libre de desordenes por deficiencia de yodo". Medicina. 2015;37(2):122-139.
- Filippini T, Malavolti M, Whelton PK, Naska A, Orsini N, Vinceti M. Blood pressure effects of sodium reduction: dose-response meta-analysis of experimental studies. Circulation. 2021;143(16):1542-1567.
doi pubmed - Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
doi pubmed - Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575-4582.
doi pubmed - Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78(1):34-44.
doi pubmed - Merrill SJ, Minucci SB. Thyroid Autoimmunity: An Interplay of Factors. Vitam Horm. 2018;106:129-145.
doi pubmed - Zaletel K, Gaberscek S. Hashimoto's thyroiditis: from genes to the disease. Curr Genomics. 2011;12(8):576-588.
doi pubmed - Kahaly GJ, Frommer L. Polyglandular autoimmune syndromes. J Endocrinol Invest. 2018;41(1):91-98.
doi pubmed - Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316.
doi pubmed - Garmendia Madariaga A, Santos Palacios S, Guillen-Grima F, Galofre JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99(3):923-931.
doi pubmed - Kasagi K, Takahashi N, Inoue G, Honda T, Kawachi Y, Izumi Y. Thyroid function in Japanese adults as assessed by a general health checkup system in relation with thyroid-related antibodies and other clinical parameters. Thyroid. 2009;19(9):937-944.
doi pubmed - Marwaha RK, Tandon N, Ganie MA, Kanwar R, Sastry A, Garg MK, Bhadra K, et al. Status of thyroid function in Indian adults: two decades after universal salt iodization. J Assoc Physicians India. 2012;60:32-36.
- Empson M, Flood V, Ma G, Eastman CJ, Mitchell P. Prevalence of thyroid disease in an older Australian population. Intern Med J. 2007;37(7):448-455.
doi pubmed - Chiovato L, Magri F, Carle A. Hypothyroidism in context: where we've been and where we're going. Adv Ther. 2019;36(Suppl 2):47-58.
doi pubmed - Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562.
doi - Kim YA, Park YJ. Prevalence and risk factors of subclinical thyroid disease. Endocrinol Metab (Seoul). 2014;29(1):20-29.
doi pubmed - Soh SB, Aw TC. Laboratory testing in thyroid conditions - pitfalls and clinical utility. Ann Lab Med. 2019;39(1):3-14.
doi pubmed - Latrofa F, Fiore E, Rago T, Antonangeli L, Montanelli L, Ricci D, Provenzale MA, et al. Iodine contributes to thyroid autoimmunity in humans by unmasking a cryptic epitope on thyroglobulin. J Clin Endocrinol Metab. 2013;98(11):E1768-1774.
doi pubmed - Ralli M, Angeletti D, Fiore M, D'Aguanno V, Lambiase A, Artico M, de Vincentiis M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19(10):102649.
doi pubmed - Benvenga S, Elia G, Ragusa F, Paparo SR, Sturniolo MM, Ferrari SM, Antonelli A, et al. Endocrine disruptors and thyroid autoimmunity. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101377.
doi pubmed - Goitre as a determinant of the prevalence and severity of iodine deficiency disorders in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2014 WHO/NMH/NHD/MNM/14.5.
- Alexander LF, Patel NJ, Caserta MP, Robbin ML. Thyroid ultrasound: diffuse and nodular disease. Radiol Clin North Am. 2020;58(6):1041-1057.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


