| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Letter to the Editor
Volume 13, Number 12, December 2021, pages 572-574
Clostridiodes difficile Treatment Guided by Polymerase Chain Reaction Stool Testing Does not Alter Outcomes for Patients With Inflammatory Bowel Disease
Ceena Chandrabosa , Kana Chinb
, Yan Liuc
, Nina Kohnd
, Arun Swaminathe
, Keith Sultanf, g
aDepartment of Medicine, Northwell Health North Shore University Hospital, 300 Community Dr., Manhasset, NY 11030, USA
bDepartment of Medicine, Northwell Health Forest Hills Hospital, 102-01 66th Rd, Forest Hills, NY 11375, USA
cBioinformatics, Feinstein Institute for Medical Research, 350 Community Dr, Manhasset, NY 11030, USA
dBiostatistics, Feinstein Institute for Medical Research, 350 Community Dr, Manhasset, NY 11030, USA
eDivision of Gastroenterology, Northwell Health Lenox Hill Hospital, 100 East 77th St., New York, NY 10075, USA
fDivision of Gastroenterology, Northwell Health North Shore University Hospital, Manhasset, NY 11021, USA
gCorresponding Author: Keith Sultan, Division of Gastroenterology, Northwell Health North Shore University Hospital, Manhasset, NY 11021, USA
Manuscript submitted September 30, 2021, accepted October 19, 2021, published online December 28, 2021
Short title: C. difficile by PCR and Treatment Outcomes in IBD
doi: https://doi.org/10.14740/jocmr4610
| To the Editor | ▴Top |
The management of Clostridioides difficile (C. difficile) infection (CDI) for patients with inflammatory bowel disease (IBD) is an ongoing challenge. CDI complicating IBD has been shown to be associated with worse outcomes including hospitalization, colectomy and death. The recent American Gastroenterological Association clinical practice review of IBD and CDI provides a comprehensive update of the factors leading to CDI with IBD, and standards of care for treatment [1]. More recently changes in recommendations for CDI treatment in the general population have been made, advancing fidaxomicin as the preferred first-line treatment, though there is not yet an updated guideline for those with IBD [2].
The essential first step to effective treatment of CDI is to make an accurate diagnosis. While this may seem simple enough, it is understood that testing with the rapid and popular high-sensitivity nucleic acid amplification test (NAAT), also known as polymerase chain reaction (PCR), may in fact over-diagnose CDI, i.e., a positive PCR may reflect C. difficile colonization rather than true infection. This potential for PCR over-diagnosing CDI has been observed in the general population, leading to recent guideline recommendations for a stepwise diagnostic approach [3, 4]. The guidelines recommend first-line testing with a high-sensitivity glutamate dehydrogenase (GDH) assay. A positive finding is followed by a more specific test directly for C. difficile toxin. Utilizing this strategy, a PCR test is only employed as an arbiter to rule in CDI for those cases with discordant results, i.e., a positive GDH with negative toxin assay.
Only recently however has real world clinical evidence emerged demonstrating a superiority of a test and treat strategy directed by toxin-positive assay over PCR testing for suspected CDI in the IBD population. Gupta et al in a study of 92 IBD patients (61% Crohn’s disease), of whom 28 (30%) where toxin-positive, found that 82% of toxin-positive patients responded to antibiotics directed against CDI compared to only 25% of toxin-negative PCR-positive patients (P < 0.001), and that only 21 % of toxin-positive patients required IBD therapy escalation compared to 63% of toxin-negative PCR-positive patients (P < 0.001) [5].
While Gupta et al’s work supports a toxin-positive result as the preferred modality of diagnosing CDI in the IBD population, it does not exclude the possibility that first-line PCR testing may still have some value and impact outcomes in the IBD population. Though guidelines clearly advocate against first-line PCR testing, this modality remains popular, widely available, and may in fact be the only test available at some institutions [6]. As such, we investigated whether patients with IBD treated for CDI based on a positive PCR had different outcomes compared to those with a negative PCR. We analyzed all outpatients 18 years and older within our health system over the last 14 years with IBD and outpatient C. difficile PCR testing with at least 1 year of documented office follow-up. C. difficile PCR-positive patients were matched 1:1 to PCR-negative patients by age, IBD type, disease extent, disease duration, and IBD therapy. Our primary outcomes compared PCR-positive and -negative patients by escalation of IBD treatment and time to escalation within 1 year following C. difficile testing. Escalations were defined as an addition/change of medications, increase in dose/frequency of current therapy, or surgery.
Of 168 IBD patients who were C. difficile PCR-positive, 46 patients met inclusion criteria. Seventy percent of patients were treated with vancomycin, and the rest with metronidazole or fidaxomicin. From 2,321 IBD patients who were C. difficile PCR-negative, 46 matches were identified. C. difficile PCR-positive patients were older than C. difficile PCR-negative patients, but had similar disease duration (Table 1). At 1-year follow-up, there was no difference between the two groups with regard to overall escalation of IBD treatment, or addition/change of biologic therapy. There was also no significant difference of time to escalation of IBD treatment within 1 year between groups at any time point (P < 0.7319) (Fig. 1).
 Click to view | Table 1. Patient Demographics and Outcomes |
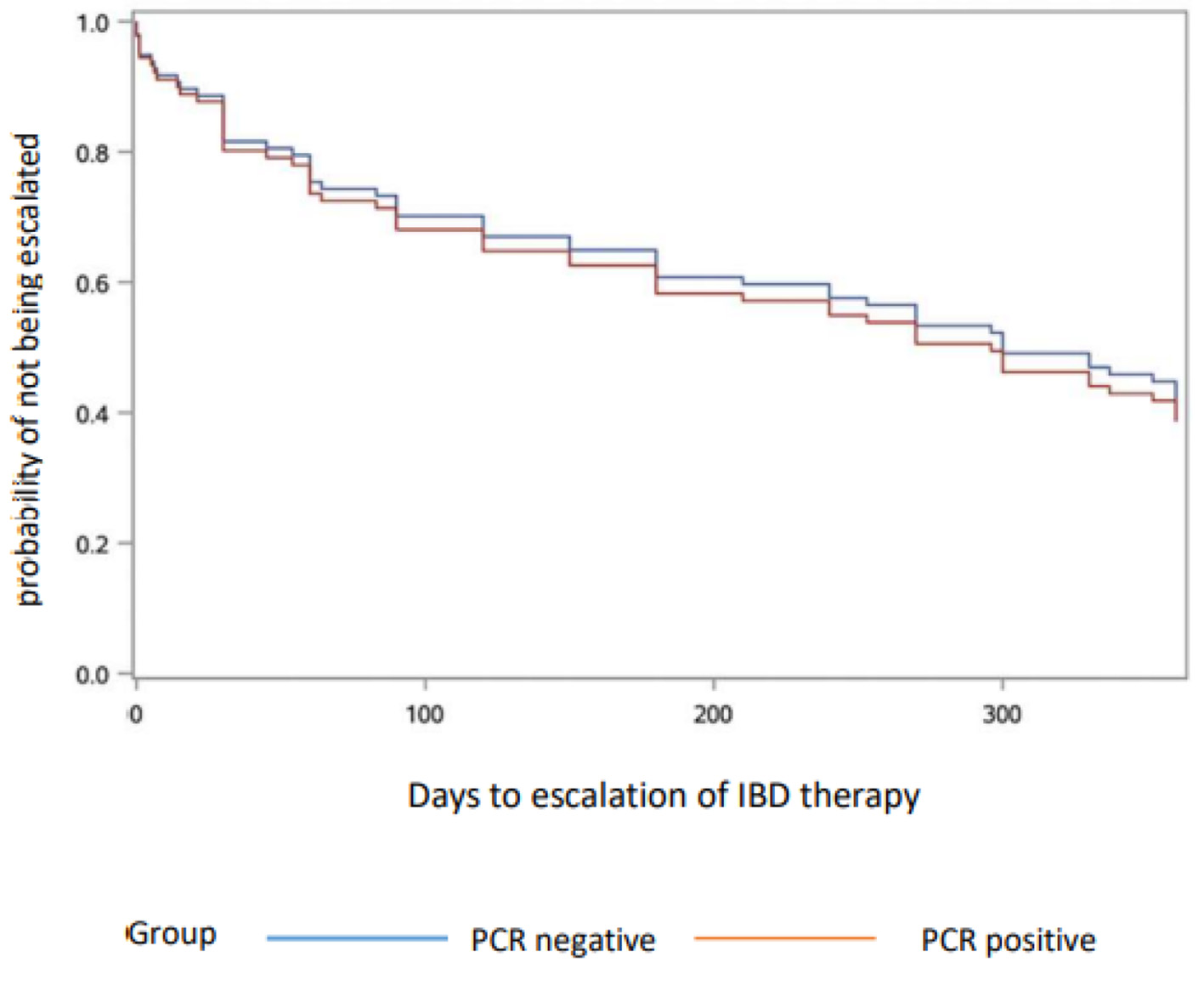 Click for large image | Figure 1. Escalation of IBD therapy. PCR: polymerase chain reaction; IBD: inflammatory bowel disease. |
Our results show that the diagnosis and treatment of CDI by a stand-alone PCR-directed strategy did not impact the clinical course in a cohort of IBD patients. In this regard, our findings add to those of Gupta et al by demonstrating that PCR testing as a guide to CDI therapy is not just inferior to toxin, but may perhaps be of no use whatsoever. It suggests that PCR-positive testing for C. difficile in the setting of IBD overwhelmingly represents patients with colonization rather than infection, making the use of toxin testing critical to distinguish between the two. Our observation of similar rates of escalation of IBD therapy between the C. difficile PCR-positive and PCR-negative IBD patients lends further real-world data to support the current stepwise testing guidelines. In accordance with these results, institutions should routinely offer stepwise GDH C. difficile testing and redirect requests away from PCR stool assays for those patients with IBD.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Requirement for informed consent was waived by IRB approval.
Author Contributions
Conceptualization: CC and KS. Methodology: CC, KS, and NK. Formal analysis: CC, KS, and NK. Data curation: CC, KC, and YU. Project administration: CC. Visualization: CC, KS, NK, and AS. Writing-original draft: CC. Writing-review and editing: KS and AS. Approval of final manuscript: all authors.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Khanna S, Shin A, Kelly CP. Management of clostridium difficile infection in inflammatory bowel disease: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15(2):166-174.
doi pubmed - Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):755-757.
doi pubmed - Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, et al. Overdiagnosis of clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175(11):1792-1801.
doi pubmed - McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48.
doi pubmed - Gupta A, Wash C, Wu Y, Sorrentino D, Nguyen VQ. Diagnostic modality of clostridioides difficile infection predicts treatment response and outcomes in inflammatory bowel disease. Dig Dis Sci. 2021;66(2):547-553.
doi pubmed - Kamboj M, Brite J, Aslam A, Kennington J, Babady NE, Calfee D, Furuya Y, et al. Artificial differences in clostridium difficile infection rates associated with disparity in testing. Emerg Infect Dis. 2018;24(3):584-587.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


