| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 2, February 2021, pages 107-112
Active Head Auto-Rotations in Patients With Benign Paroxysmal Positional Vertigo
Sertac Yetisera, b, Dilay Incea
aAnadolu Medical Center, Dept of ORL & HNS, Gebze, Kocaeli 41400, Turkey
bCorresponding Author: Sertac Yetiser, Anadolu Medical Center, Dept of ORL & HNS, Gebze, Kocaeli 41400, Turkey
Manuscript submitted December 14, 2020, accepted December 30, 2020, published online February 25, 2021
Short title: Active Head Auto-Rotations in Patients With BPPV
doi: https://doi.org/10.14740/jocmr4413
| Abstract | ▴Top |
Background: Utricular degeneration is the source of traveling otoconia inside the semicircular canals in patients with benign paroxysmal positional vertigo (BPPV). The underlying pathology is not clear. The aim of this study was to analyze vestibulo-ocular reflex (VOR) during sudden head accelerations in those patients since clinical reports designating an association of BPPV with inner ear problems are increasing.
Methods: VOR reaction to impulsive head rotations were tested in 34 patients with BPPV (13 lateral, 21 posterior canal BPPV) and 15 healthy subjects in a prospective controlled study. Main outcome measure was the gain (the ratio of head and eye velocity) of vertical and horizontal head auto-rotations to the pathologic and normal sides.
Results: All patients with BPPV and control subjects had normal gain (≥ 0.9) at 1 and 2 Hz but the gain decreased at higher frequencies. No statistically significant difference was found when comparing the gain between the horizontal head rotations toward the pathologic and those toward the normal side (P = 0.89, P = 0.90, P = 0.78, P = 0.20 and P = 0.16, at 1, 2, 3, 4 and 5 Hz, respectively) and between upward and downward vertical head rotations (P = 0.28, P = 0.53 and P = 0.15, at 1, 2 and 3 Hz, respectively) in patients with lateral and posterior canal BPPV.
Conclusion: VOR gain was reduced in some patients. However, head auto-rotation test (HART) does not show any functional abnormality of VOR during head rotations toward the pathologic side. HART is not suitable as a screening test for BPPV.
Keywords: Head auto-rotation; Benign paroxysmal positional vertigo; Nystagmus; Vestibulo-ocular reflex
| Introduction | ▴Top |
Semicircular canals are activated during rapid head movements and otolithic signal is created to provide information to central structures for the direction of motion and the orientation of the body [1]. Investigation of the vestibular end organ in patients with benign paroxysmal positional vertigo (BPPV) is important to understand the underlying pathophysiology. When the vestibulo-ocular reflex (VOR) is functioning normally, vestibular system generates compensatory eye movements in the opposite direction to the rapid head rotation. VOR is the fastest reflex system in the body and operates in 5 to 7 ms. In case of a deficit in the VOR, the eyes are not able to look at the intended target at the end of head movement and images shift on the fovea, momentarily. The ratio of head and eye velocity (the gain) decreases. Head auto-rotation test (HART) allows studying the vestibular function starting from lower frequencies sweeping up to higher frequencies over a physiologic range and provides earlier and more information than other conventional tests [2, 3]. Caloric testing is considered the gold standard and is routinely performed. However, HART helps to clarify the impact of otolithic dysfunction on VOR during high acceleration impulses.
Patients with BPPV experience a sudden sense of spinning in daily life when they turn their head to either side or when they bend their head forward or backward due to freely moving otoconia inside the semicircular canals in response to sudden head motion [4]. A brief spontaneous positional nystagmus is associated with these conditions which may eventually impair patients’ stability temporarily. BPPV is associated with several inner ear diseases, but normal VOR function is expected in those patients since no specific organic pathology has been demonstrated. However, utricular degeneration leading to otoconial detachment is blamed as the source of traveling crystalloids. Patients are usually quite well after treatment. But some people may have vestibular instability even after disappearance of positional nystagmus and vertigo [5, 6]. Vestibular function in patients with BPPV in acute setting has not been studied and how well this reflex system works in those patients is not clear. The aim of this study is to analyze the gain (the ratio of head and eye velocity) of the head acceleration to the pathologic and normal sides in 34 patients with BPPV of posterior and lateral canal in a controlled study.
| Materials and Methods | ▴Top |
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board. The study was completed in a regional community health center. Thirty-four patients with BPPV (21 posterior, 13 lateral canal; four ageotropic, nine geotropic type) who have been examined at outpatient clinic between 2017 - 2019 were enrolled for the study. All patients were consecutive. Duration of symptoms was ranging between 3 and 42 days (12.08 ± 11.57 days). Thirteen males and 21 females (ratio: 1.76), aged between 24 and 57 were included (39.06 ± 9.23). All patients were tested at the day of initial admission. Fifteen healthy participants (eight males and seven females; ratio: 1.14) with no history of vertigo, aged between 37 and 52 years old were enrolled (41.02 ± 3.34). All subjects had normal otoscopic view and normal hearing threshold. None of the subjects had a history of hearing loss, head or ear surgery. Those with visual disturbances, systemic problems, oculomotor diseases, cervical spine lesions, and mental diseases and those having any medication 1 week prior to test which may have an impact on vestibular system were excluded. A commercially available system was used for head rotation testing (Vortaq, Micromed, Inc., Chatham, IL, USA). It was performed by eye movement recording with goggles having infra-red wireless camera system. Head movement was simultaneously recorded in the vertical and horizontal planes by a sensor affixed into the holder on the binocular goggle. There were two test conditions, including horizontal (yaw; head shaking) and vertical head auto-rotations (pitch; head nodding).
Patients were tested in the illuminated room sitting 100 cm away from laptop computer at the level of their eyes. Testing procedure was explained to the subject and the subject was asked to move the head side to side and then up and down (forward-flexed) with a tone played by the computer to guide the subject for the required frequency of head movements while keeping his eyes focused on the red dot on the computer. Each test was repeated for data averaging and a 2-min break was allowed between tests. Test conditions were performed randomly. The patient was tested with a frequency sweep manner between 1 and 5 Hz for the horizontal and between 1 and 3 Hz for the vertical head rotations within 15 s. After a predefined number of cycles have occurred, the frequency increases 1 Hz each time until the end frequency has been attained for at least the minimum number of cycles acceptable. The computer was set to determine the frequency of all cycles of head rotation during the test and to group them as 1 Hz (from 0.5 to 1.5 Hz), 2 Hz (from 1.5 to 2.5 Hz), 3 Hz (from 2.5 to 3.5 Hz), 4 Hz (from 3.5 to 4.5 Hz) and finally 5 Hz (from 4.5 to 5.5 Hz). Small variations in the eye signal, causing some noise and eye blinks, were smoothed by the computer. Minimum number of cycles at lowest frequency was set to 5. Minimum cycle acceptance percentage for one test was set to 50%. This parameter prevents cycles with a lot of artifacts from being included in the calculations.
Statistical analysis
Each frequency “class” was then averaged to create the patient’s average head velocity curve. In addition, an average eye velocity curve was created for each frequency class. The gain of the VOR was computed as the ratio of eye and head velocity data across each of the frequencies. Mean horizontal and vertical gains were averaged for each patient. Mean values and standard deviations (SDs) were calculated for each condition. Average gains during active horizontal head rotations to the pathologic and normal side in patients with lateral and posterior canal BPPV were grouped separately for comparative analysis. Average gains during active vertical upward and downward head rotations in patients with lateral and posterior canal BPPV were grouped for comparative analysis. Mean gain during up and down head movements in healthy subjects was accepted as vertical gain value of the controls. Mean gain during right and left head movements in healthy subjects was accepted as lateral gain value of the controls. One-way analysis of variance (ANOVA) test was used to analyze the groups with Statistical Package for the Social Sciences (SPSS 17.0 version, IBM, Chicago, IL, USA). Statistical significance was set at P < 0.05. Mean horizontal and vertical gains across testing frequencies for patients with lateral and posterior canal BPPV were also plotted.
| Results | ▴Top |
No statistical difference was found when the mean ages of the patients and control groups were compared (39.06 ± 9.23 and 41.02 ± 3.34, P = 0.83). No statistical difference was found when female and male ratio was compared between control and patient group (1.76 vs. 1.14). Recording of eye and head movement from a patient during vertical and horizontal head rotations across 1 - 5 Hz frequency is seen on Figure 1. Average gain at 1 - 5 Hz frequencies during active horizontal head rotations toward the pathologic and normal sides in patients with lateral and posterior canal BPPV is demonstrated on Table 1. All patients with BPPV and control subjects had normal gain (≥ 0.9) at 1 and 2 Hz. The gain decreased at higher frequencies in all groups. However, no statistically significant difference was found when comparing the gain between the horizontal head rotations toward the pathologic and those toward the normal side at 1, 2, 3, 4 and 5 Hz frequencies in patients with lateral and posterior canal BPPV (P = 0.89, P = 0.90, P = 0.78, P = 0.20, P = 0.16, at 1, 2, 3, 4 and 5 Hz, respectively). Average horizontal gain during active head rotations toward the healthy and pathologic sides at 1 - 5 Hz in patients with lateral and posterior canal BPPV and the control subjects is plotted in Figure 2.
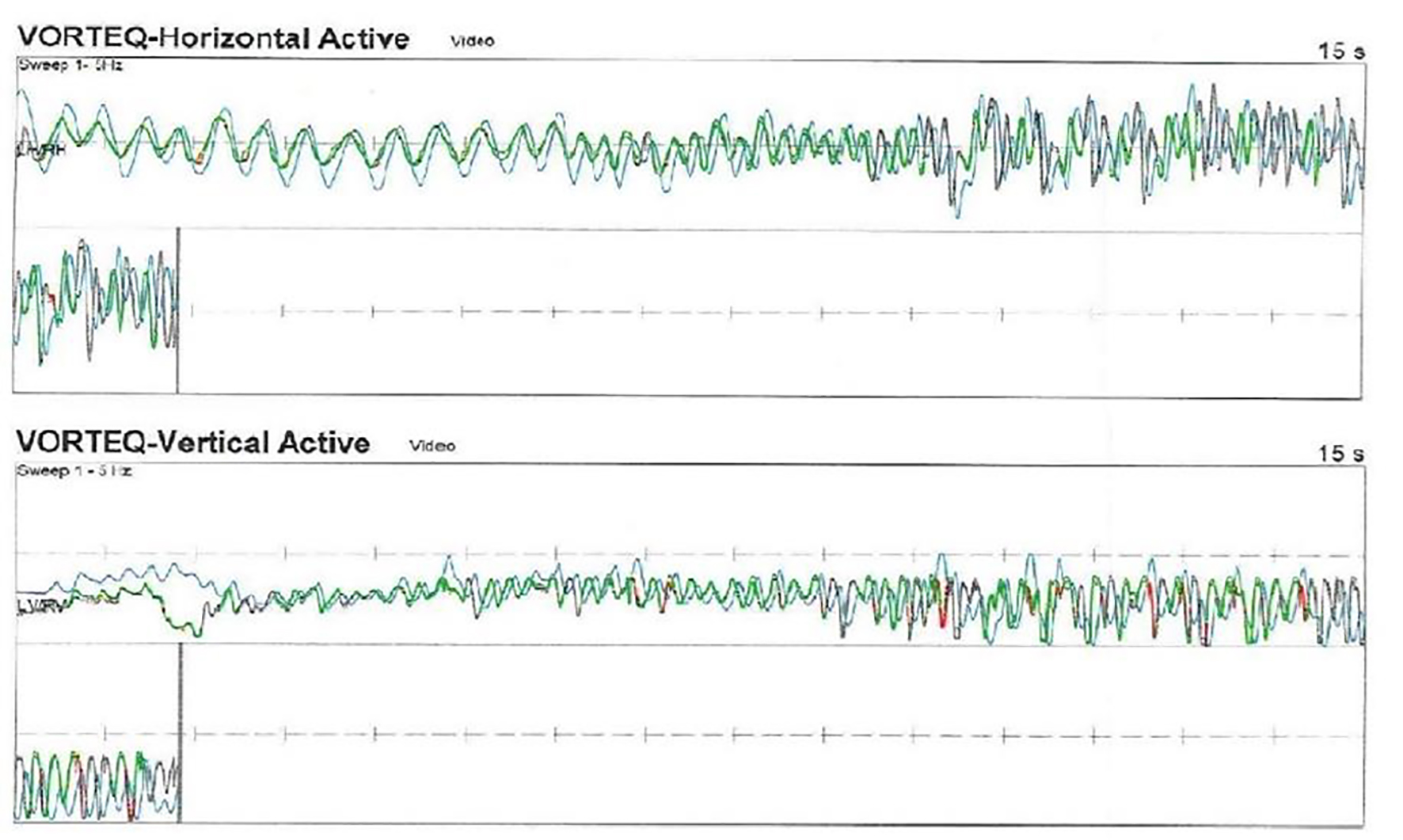 Click for large image | Figure 1. Active head auto-rotation recording of the patient with benign paroxysmal positional vertigo (BPPV). Head and eye recordings during horizontal head rotations are seen in upper drawing. Lower recording shows vertical head and eye movement (head rotations are dark, and eye movements are light color). |
 Click to view | Table 1. Average Gains at 1 - 5 Hz Frequencies During Active Horizontal Head Rotations Toward the Pathologic and Normal Sides in Patients With Lateral and Posterior Canal BPPV |
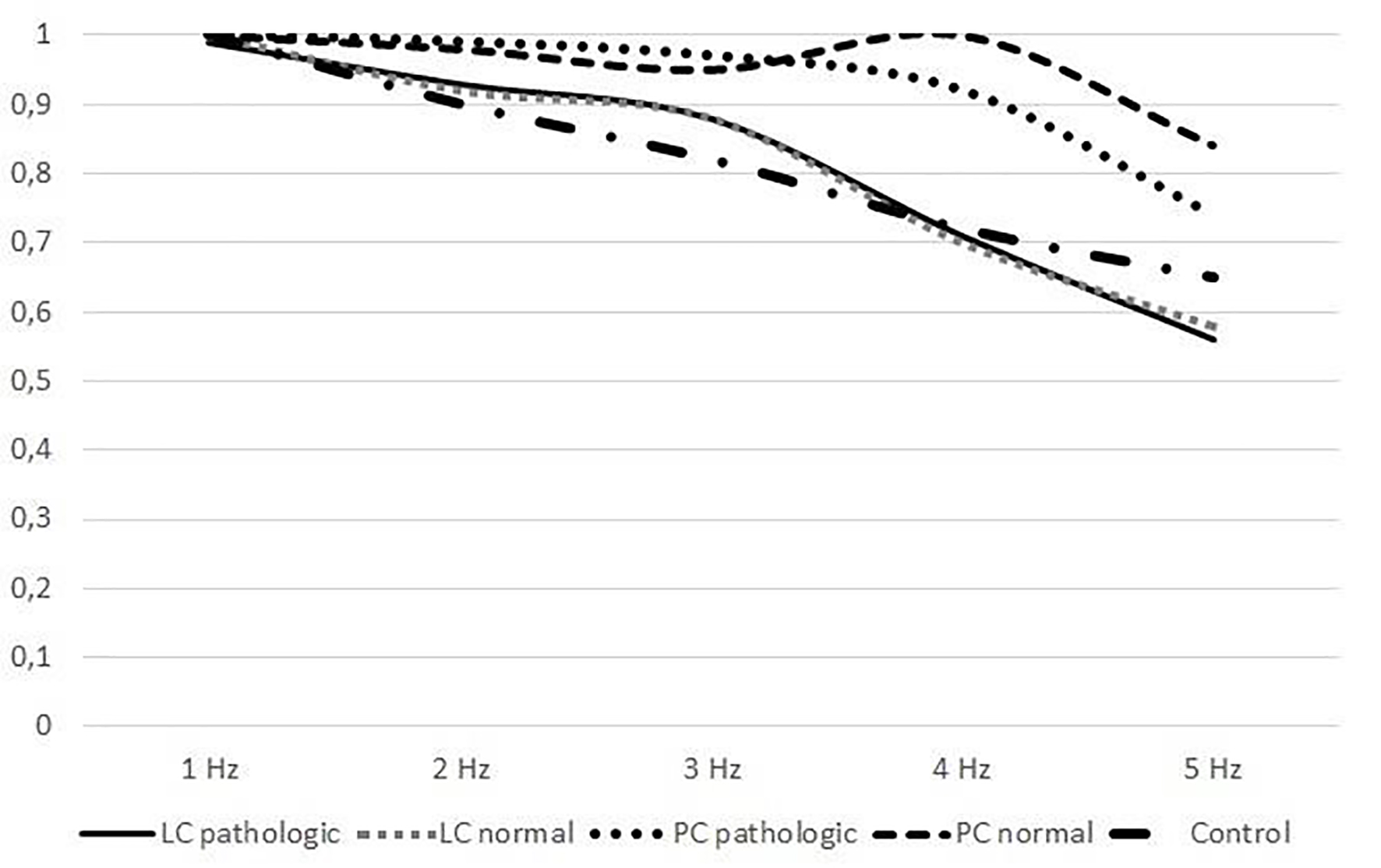 Click for large image | Figure 2. Comparison of horizontal gain in patients with BPPV and control subjects (LC: lateral canal, PC: posterior canal). |
Average gain at 1, 2 and 3 Hz during vertical head rotations was lower both in normal subjects and in patients with lateral and posterior canal BPPV (Table 2). However, no statistically significant difference was found when comparing the gain between upward and downward vertical head rotations at 1, 2 and 3 Hz frequencies in patients with lateral and posterior canal BPPV (P = 0.28, P = 0.53 and P = 0.15, at 1, 2 and 3 Hz, respectively). Figure 3 shows the vertical gain at 1 - 3 Hz frequencies in patients with lateral and posterior canal BPPV and control subjects.
 Click to view | Table 2. Average Gains at 1, 2 and 3 Hz During Vertical Head Rotations in Patients With Lateral and Posterior Canal BPPV |
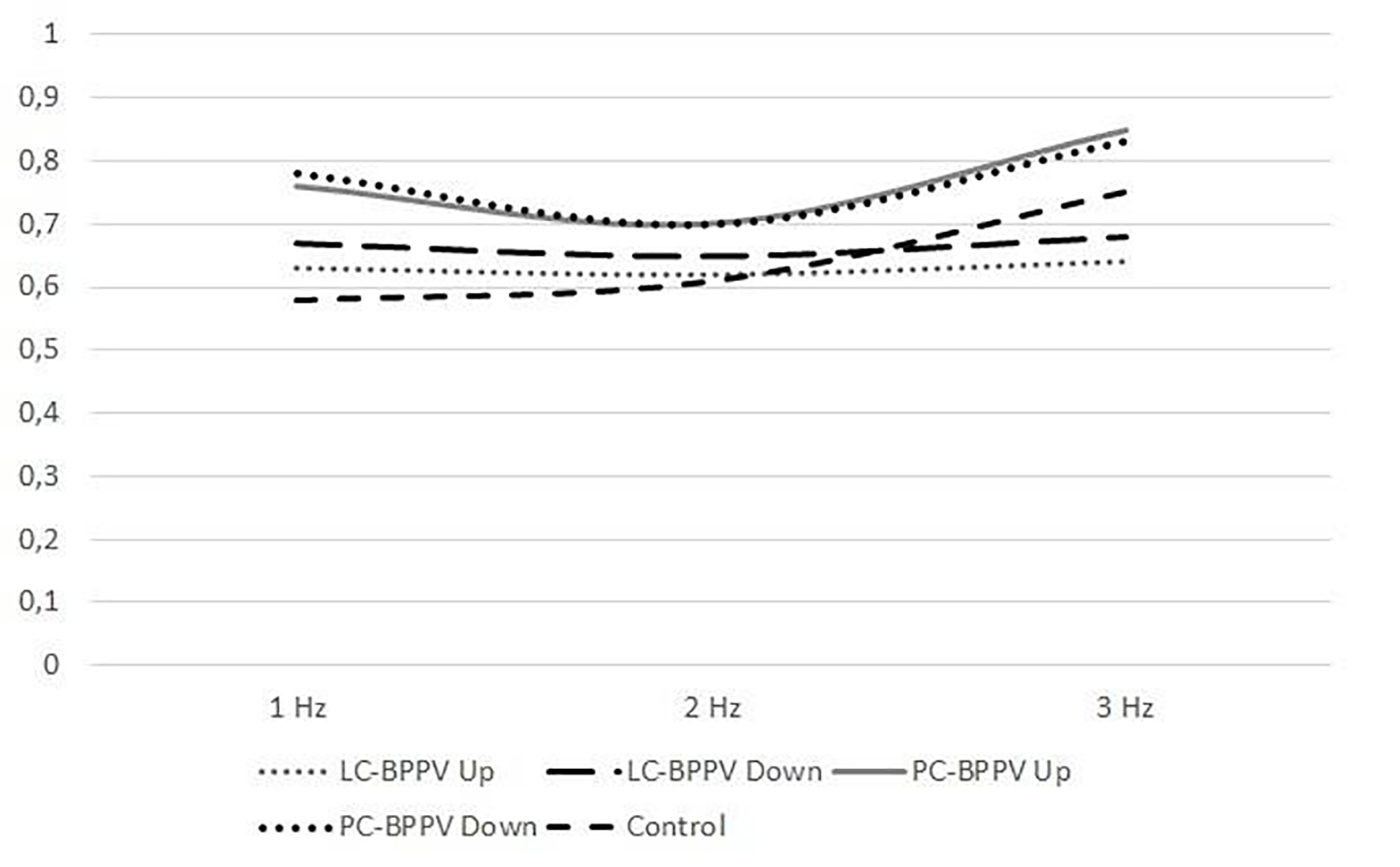 Click for large image | Figure 3. Comparison of vertical gain in patients with BPPV and control subjects (LC: lateral canal, PC: posterior canal). |
| Discussion | ▴Top |
Caloric stimulation is the basic test to evaluate the vestibular function, but it is effective on lower frequency bands and mainly measures the function of the lateral semicircular canals. HART is functional over a wide frequency spectrum and it is less affected by mental alertness as compared with electronystagmography [7, 8]. Patients with normal bithermal caloric testing may have HART abnormality [9]. HART to evaluate the vestibular function using consecutive head rotations was first introduced to clinical practice in 1988 [7]. Evaluation of eye movement response to active head oscillations at physiologic frequencies between 2 and 6 Hz in the horizontal and vertical planes while the subject is fixating a visible target provides valuable information for vestibular function. Collected data reflect the VOR function since the contribution of smooth pursuit and visual fixation systems are insignificant at these frequencies. Vestibular function is presented as gain and phase by measuring simultaneously recorded eye movements and head rotations along several frequencies.
Reliability of head auto-rotation was tested in healthy subjects. Corvera et al have found no significant differences between the inter-session and inter-individual results in 17 subjects when they were tested at an interval of 7 days [10]. HART in evaluating patients with vestibulopathy presents interesting outcomes. The test demonstrated asymmetry at high frequencies in patients with vestibular schwannoma [11, 12]. Valuable information about the functioning of VOR and the effect of intratympanic gentamicin in patients with Meniere’s disease can be obtained by HART [13-15]. Video head impulse test (VHIT) is the analog testing of VOR. HART means several consecutive head impulses but at different and increasing frequencies. Santina et al have compared the HART and VHIT in patients with unilateral vestibular deficiency and have found that HART is as effective as VHIT [16].
No specific organic pathology in patients with BPPV has been reported although several inner ear diseases can be associated with BPPV. Yet, normal vestibular function and normal VOR system are expected. VOR reflects timing or synchrony between head and eye movements and abnormal values express gaze instability and conflict between visual, vestibular and proprioceptive inputs. Therefore, it is noteworthy to investigate whether induced positional nystagmus due to impulsive head rotations may have an impact on head/eye velocity ratio. The utility of HART in the evaluation of patients with BPPV is not clear. Ozgirgin and Tarhan have reviewed the gain and phase before and after Epley maneuver and they have found no abnormality [17]. Belafsky et al have reviewed the diagnostic role of HART in 91 patients with BPPV and they have found normal horizontal gain and vertical phase lead in 87% of patients [18]. Average gain during horizontal and vertical head auto-rotations between normal subjects and patients with BPPV was not different in this study and no difference was found in gains between the head movement toward the pathologic side and toward the normal side in patients with BPPV. But the gain decreased at higher frequencies in all patients. Hirvonen et al have studied vestibulo-ocular reflex by head rotation testing in 125 healthy subjects over the frequency range of 0.5 - 5 Hz. They have proposed that upper frequency limit should be 4 Hz since the frequency band of 5 Hz was reached by 78% of the subjects, and that of 4 Hz was reached by 94% of the subjects [19].
In previous studies, HART has been investigated in patients with chronic vestibulopathy. One limitation in this study could be the fact that HART was not easy to perform in patients with acute discomfort. The test should be clearly explained to the subject to increase the learning since active participation and cooperation of the subject is necessary. Sometimes, practicing is required before recording. Stress and fear of having vertigo may prevent the patients from doing the test [20]. Another limitation is the age and testing the patients with cervical arthritis. Hirvonen et al have reported 86% abnormal HART results in healthy elder subjects [21]. Another difficulty is that the vertical head rotations can be more challenging and difficult to perform. Gaze instability and blurred vision have been reported even in normal subjects during vertical high-frequency head movements [22]. Therefore, sweep frequency of vertical head rotations was set to 1 - 3 Hz, age-limitation was applied and the patients were tested twice to get healthy average data in the presented study. Finally, electrodes may interfere with muscle activity in conventional recording techniques and this problem can only be solved with wireless infrared camera system [23].
In conclusion, vestibular function in patients with BPPV during their first visit to ear, nose, and throat (ENT) outpatient clinic was not investigated before. VOR was not abnormal as tested by HART. Testing the patients with BPPV with head auto-rotation has some limitations. However, the test was well tolerated by the patients even though they had acute balance problem. The gain was lower in some patients. However, no statistically significant difference was found when comparing the gain between head rotations toward the pathologic and toward the normal side in patients with lateral and posterior canal BPPV.
Acknowledgments
None to declare.
Financial Disclosure
This study has no grant or funding.
Conflict of Interest
The authors declare that they have no conflict of interest in the manuscript.
Informed Consent
An informed consent was obtained from all individual participants included in the study.
Author Contributions
The concept of the study was designed by Dr S. Yetiser and Mrs D. Ince. The data were collected by Mrs Ince and were analyzed and interpreted by Dr S. Yetiser. The study was revised and final approval of the integrity and accuracy was made by Dr S. Yetiser and Mrs Ince.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Lempert T, Gianna CC, Gresty MA, Bronstein AM. Effect of otolith dysfunction. Impairment of visual acuity during linear head motion in labyrinthine defective subjects. Brain. 1997;120(Pt 6):1005-1013.
doi pubmed - Hsieh LC, Lin TM, Chang YM, Kuo TB, Lee GS. Clinical applications of correlational vestibular autorotation test. Acta Otolaryngol. 2015;135(6):549-556.
doi pubmed - O'Leary DP, Davis LL. Spectral analysis of low-frequency, active-head vestibulo-ocular reflex responses. J Vestib Res. 1998;8(4):313-324.
doi pubmed - Bhattacharyya N, Baugh RF, Orvidas L, Barrs D, Bronston LJ, Cass S, Chalian AA, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47-81.
doi pubmed - Sen A, Al-Deleamy LS, Kendirli TM. Benign paroxysmal positional vertigo in an airline pilot. Aviat Space Environ Med. 2007;78(11):1060-1063.
doi pubmed - Lee NH, Kwon HJ, Ban JH. Analysis of residual symptoms after treatment in benign paroxysmal positional vertigo using questionnaire. Otolaryngol Head Neck Surg. 2009;141(2):232-236.
doi pubmed - Kitsigianis GA, O'Leary DP, Davis LL. Active head-movement analysis of cisplatin-induced vestibulotoxicity. Otolaryngol Head Neck Surg. 1988;98(1):82-87.
doi pubmed - Pulaski PD, Zee DS, Robinson DA. The behavior of the vestibulo-ocular reflex at high velocities of head rotation. Brain Res. 1981;222(1):159-165.
doi - Saadat D, O'Leary DP, Pulec JL, Kitano H. Comparison of vestibular autorotation and caloric testing. Otolaryngol Head Neck Surg. 1995;113(3):215-222.
doi - Corvera J, Corvera-Behar G, Lapilover V, Ysunza A. Evaluation of the vestibular autorotation test (VAT) for measuring vestibular oculomotor reflex in clinical research. Arch Med Res. 2000;31(4):384-387.
doi - Hirvonen TP, Aalto H, Pyykko I. Decreased vestibulo-ocular reflex gain of vestibular schwannoma patients. Auris Nasus Larynx. 2000;27(1):23-26.
doi - O'Leary DP, Davis LL, Maceri DR. Vestibular autorotation test asymmetry analysis of acoustic neuromas. Otolaryngol Head Neck Surg. 1991;104(1):103-109.
doi pubmed - Perez N, Martin E, Garcia-Tapia R. Results of vestibular autorotation testing at the end of intratympanic gentamicin treatment for Meniere's disease. Acta Otolaryngol. 2003;123(4):506-514.
doi pubmed - Hirvonen TP, Pyykko I, Aalton H. A head rotation test for patients with Meniere's disease. Auris Nasus Larynx. 1998;25:111-119.
doi - Ng M, Davis LL, O'Leary DP. Autorotation test of the horizontal vestibulo-ocular reflex in Meniere's disease. Otolaryngol Head Neck Surg. 1993;109(3 Pt 1):399-412.
doi pubmed - Della Santina CC, Cremer PD, Carey JP, Minor LB. Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg. 2002;128(9):1044-1054.
doi pubmed - Ozgirgin ON, Tarhan E. Epley maneuver and the head autorotation test in benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2008;265(11):1309-1313.
doi pubmed - Belafsky P, Gianoli G, Soileau J, Moore D, Davidowitz S. Vestibular autorotation testing in patients with benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2000;122(2):163-167.
doi - Hirvonen TP, Pyykko I, Aalto H, Juhola M. Vestibulo-ocular reflex function as measured with the head autorotation test. Acta Otolaryngol. 1997;117(5):657-662.
doi pubmed - Blatt PJ, Schubert MC, Roach KE, Tusa RJ. The reliability of the Vestibular Autorotation Test (VAT) in patients with dizziness. J Neurol Phys Ther. 2008;32(2):70-79.
doi pubmed - Demer JL, Oas JG, Baloh RW. Visual-vestibular interaction in humans during active and passive, vertical head movement. J Vestib Res. 1993;3(2):101-114.
- Hirvonen TP, Aalto H, Pyykko I, Juhola M, Jantti P. Changes in vestibulo-ocular reflex of elderly people. Acta Otolaryngol Suppl. 1997;529:108-110.
doi pubmed - Guyot JP, Psillas G. Test-retest reliability of vestibular autorotation testing in healthy subjects. Otolaryngol Head Neck Surg. 1997;117(6):704-707.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


