| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 12, Number 12, December 2020, pages 753-757
Mechanism and Effect of Beta-Blockers on Pancreatic Adenocarcinoma: A Literature Review
Vandan D. Upadhyayaa, f, Steven Douedia, Brandon Garciab, Jimmy Gonzalezc, Ndausung Udongwoa, Juanqin Weid, Qiang Naie, Arif Asifa, Shuvendu Sena
aDepartment of Internal Medicine, Hackensack Meridian Health Jersey Shore University Medical Center, Neptune, NJ, USA
bDepartment of Pharmacy, Hackensack Meridian Health Jersey Shore University Medical Center, Neptune, NJ, USA
cErnest Mario School of Pharmacy, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA
dRobert Wood Johnson University Hospital - Somerset, Somerville, NJ, USA
eMassachusetts General Cancer Center, Cooley Dickinson Hospital, Northampton, MA, USA
fCorresponding Author: Vandan Upadhyaya, Department of Internal Medicine, Hackensack Meridian Jersey Shore University Medical Center, Neptune, NJ 07753, USA
Manuscript submitted October 29, 2020, accepted November 24, 2020, published online December 18, 2020
Short title: Beta-Blockers on Pancreatic Adenocarcinoma
doi: https://doi.org/10.14740/jocmr4387
- Abstract
- Introduction
- Beta-Adrenergic Receptor Physiology
- Beta-Adrenergic Pathways in Pancreatic Ductal Adenocarcinoma Cell (PDAC)
- Current Outcomes on Beta-Blocker Use in Pancreatic Adenocarcinoma
- Conclusion
- References
| Abstract | ▴Top |
Pancreatic adenocarcinoma has a poor 5-year survival rate despite many advancements in pharmacotherapies. Studies have suggested the involvement of β-adrenergic pathway in the progression of pancreatic adenocarcinoma. Animal experiments and retrospective trials have reported the use of beta-blockers as potential chemo-preventative agents. This review aims to discuss β-adrenergic physiology as it relates to the progression of pancreatic adenocarcinoma and review outcomes on the use of beta-blockers for its treatment.
Keywords: Beta-adrenergic pathway; Pancreatic adenocarcinoma; Beta-blockers; Cancer
| Introduction | ▴Top |
Cancer is the second leading cause of death worldwide, with pancreatic cancer ranked as the seventh leading cause of cancer-related mortality worldwide and third in the United States [1, 2]. There are two types of pancreatic cancer, adenocarcinoma being the most common and pancreatic neuroendocrine tumor accounting for less than 10% of cases [2]. Despite advancements in medicine and increased awareness, pancreatic adenocarcinoma 5-year survival rate stands at a mere 9% [2, 3]. This significantly high mortality rate has been attributed to delayed diagnosis due to presenting symptoms found more in advanced disease and delayed treatment plans [2].
The incidence of pancreatic cancer increases with age and varies depending on geographic location [3]. Risk factors can be divided into modifiable, such as smoking, obesity and alcohol use (defined as greater than three drinks per day), and non-modifiable such as gender (males greater than females), age, ethnicity, genetics and diabetes mellitus [2-6].
Beta-blocker medications, such as propranolol, have been suggested to improve cancer prevention and relapse [7, 8]. This assumption has been increasingly focused around breast cancer where several studies demonstrated decreased Bcl-2 markers and increased p53 protein markers in patients taking propranolol, a non-selective beta-blocker [7, 9]. Furthermore, studies have shown non-selective beta-blockers decrease the risk of cancer metastasis, specifically in the breast cancer population [10-12]. In pancreatic cancer, studies have shown that the induction of the sympathetic nervous system can cause an increase in catecholamines which in turn can increase malignant cell proliferation [7, 13-16]. Blocking the activity of these beta-adrenergic receptor pathways has been associated with improved survival outcomes in patients with pancreatic cancer [7, 17]. Although still poorly understood, and while no concrete association has been developed yet, an increasing number of clinical trials are underway to help confirm this association. In this article, we aim to aid in the understanding of the biochemical pathways of which beta-blockade may decrease pancreatic cell proliferation and help improve survival and outcomes of a deadly and increasingly prevalent malignancy. The primary focus of this review will be addressing the involvement of β2-adrenergic receptor (β2AR) pathway in pancreatic ductal adenocarcinoma and review associated effects of its blockade.
| Beta-Adrenergic Receptor Physiology | ▴Top |
Adrenergic receptors are types of G-protein-coupled receptors (GPCRs), the largest class of transmembrane signaling proteins among vertebrates. All GPCRs share similar structural homology, including a characteristic domain of seven transmembrane, hydrophobic, alternating intra- and extracellular looped α-helices and a coupled heterotrimeric G-protein. The heterotrimeric G-protein is composed of an α, β and γ subunit of which the α-subunit exerts GTPase activity; GPCRs induce intracellular signaling pathways in response to hormones, neurotransmitters and other stimuli by dissociation of the α- and βγ subunits. Various isoforms of α-subunit (e.g., Gs, Gi and Gq) may interact with adenylate cyclase (AC) and calcium channels (among others) to propagate second messenger signaling [18].
Adrenergic receptors are subdivided into two major classes, α- and β-receptors, each of which contains subdivisions [19]. Of the three types of β-receptors, the authors of this review will primarily focus on β2AR. The β2AR normally binds epinephrine and norepinephrine and is predominantly concentrated airway smooth muscle and cardiac tissue [19, 20]. However, distribution in other tissues such as the pancreas has been identified more recently [21]. Ligand binding to the β2AR leads to activation and dissociation of the Gαs and βγ subunits. The Gαs subunit activates AC to catalyze formation of cyclic adenosine monophosphate (cAMP) and thus promotes the activity of protein kinase A (PKA). cAMP may also inhibit release of calcium ions from intracellular stores.
The β2AR may also couple to Gi proteins following PKA-mediated phosphorylation of the receptor itself. This process stimulates p38 mitogen-activated protein kinase (MAPK) pathways using the βγ subunits as a scaffold for intracellular protein assembly of SRc, Raf and RAS. The downstream effect may be MAPK phosphorylation of the glucocorticoid receptor (GR) and activation of the CCAAT enhancer binding protein (C/EBPα) [20].
| Beta-Adrenergic Pathways in Pancreatic Ductal Adenocarcinoma Cell (PDAC) | ▴Top |
Beta-adrenoceptors have been found to be expressed by PDACs, and play a role within tumor invasion, proliferation and inhibition of apoptosis [8, 14, 22, 23]. While both β1 and β2 adrenoceptors have been identified, it has been found that β2ARs in particular have been implicated in the growth and invasion of PDAC in vitro [14, 15]. Several complex receptor-controlled signal transduction pathways have been shown to play a role in β-mediated PDAC transcription and DNA synthesis.
Cell proliferation is thought to be stimulated by β-adrenergic receptor activation via the activation of the PKA pathways [16]. PKA signaling can induce proliferation and prevent apoptosis through the cAMP response element binding protein (CREB), activator protein 1 (AP-1) or NF-kB [16]. The epidermal growth factor receptor (EGFR) is also activated by PKA signaling and release of endothelial growth factor by cAMP. Activation of the EGFR leads to downstream signaling mediated by the Ras/Raf/ERK 1/2 pathway, stimulating further proliferation [16]. Additionally, activation of the P38/MAPK pathway has been shown to stimulate cell growth and prevent apoptosis in PDAC [15, 24-26].
Beta-adrenergic receptor activation is also associated with increased concentrations of matrix metalloproteinases (MMP-2 and MMP-9) and vascular endothelial growth factor (VEGF) [13, 22, 23]. Increased expression and production of MMP-2 and MMP-9 allow degradation of extra-cellular matrix macromolecules and cell adhesion molecules, as well as promote release and activation of growth factors, enhancing tumor invasion and proliferation [26-28]. Release of VEGF drives angiogenesis, a process critical for tumor survival and growth [22, 28]. Overall, the β-adrenergic system may potentiate tumor growth, spread and survival by a plethora of signal transduction pathways resulting in upregulation of gene expression and transcription (Fig. 1) [29].
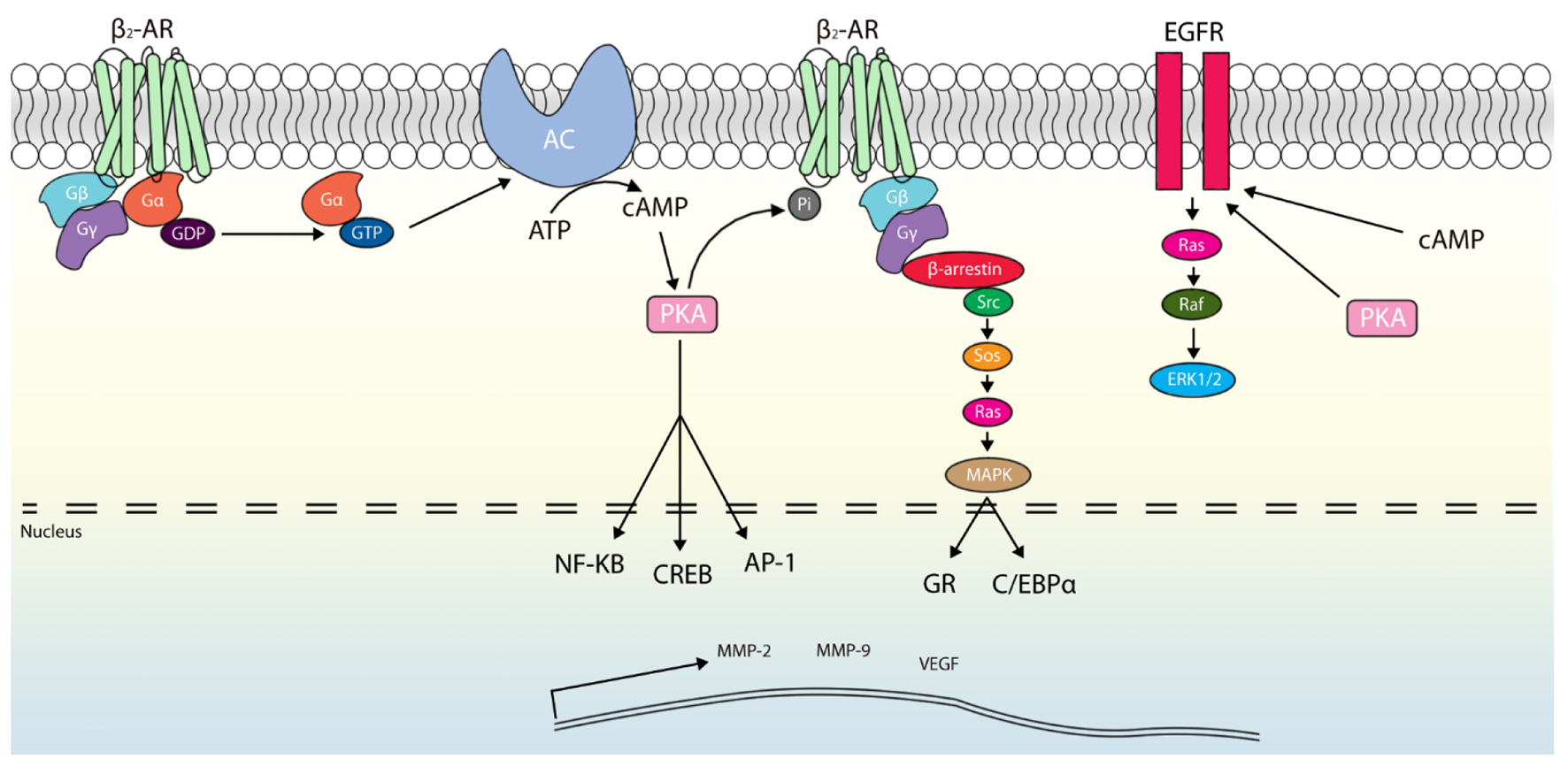 Click for large image | Figure 1. Pathways of adrenergic-mediated oncogenesis within pancreatic ductal cells. Activation of the β2-adrenergic receptor (β2AR) leads to α-subunit binding with guanosine triphosphate (GTP) and release. Activated Gα can promote adenylate cyclase (AC) activity, which produces cyclic adenosine monophosphate (cAMP). Protein kinase A (PKA) is then activated by cAMP, which in turn stimulates generation of multiple transcription factors (NF-kB, cAMP response element binding protein (CREB) and activator protein 1 (AP-1)). Additionally, β2AR may be phosphorylated by PKA to serve as scaffolding for β-arrestin and to initiate a second messenger cascade culminating in p38 mitogen-activated protein kinase (MAPK)-mediated activation of glucocorticoid receptor (GR) and CCAAT enhancer binding protein (C/EBPα). Similarly, an epidermal growth factor receptor (EGFR)-mediated pathway can be promoted through cAMP and PKA activity downstream of β2AR. These signaling cascades lead to transcription of proliferation and angiogenesis promoters (e.g., matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor (VEGF)). |
| Current Outcomes on Beta-Blocker Use in Pancreatic Adenocarcinoma | ▴Top |
As discussed above, β-adrenergic pathway has been implicated in the clinical course of pancreatic ductal adenocarcinoma [15]. Weddle et al sought to study the β-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinoma. Studies have shown overexpression of cyclooxygenase-2 and 5-lipoxygenase in exocrine pancreatic carcinomas [30, 31], suggesting a potential role of arachidonic acid in the malignancy. Weddle et al found high basal levels of arachidonic acid levels release in human cell lines derived from exocrine ductal pancreatic carcinoma and expressed β2 and β1 adrenergic receptors [15]. Further observation found that nicotine-derived nitrosamine ketone (NNK), a known inducer of pancreatic ductal adenocarcinoma in animal studies [32, 33], expressed β-adrenergic activity in cell lines and expressed higher levels of arachidonic acid. Compared to controls and NNK-induced cell lines, basal release of arachidonic acid exposed to β-adrenergic antagonist was significantly reduced. Weddle et al suggested the implication of the use of β-blockers as a part of clinical management towards chemo-preventative strategies [15].
Live animal studies performed by Al-Wadei et al suggested similar findings to Weddle et al [15]. Al-Wadei et al utilized three groups of 12 golden hamsters pretreated prenatally with ethanol only, ethanol with NNK, and ethanol with NNK and propranolol and compared the development of pancreatic ductal adenocarcinoma based on America Joint Committee of Cancer histopathological classification [33]. All hamsters treated transplacentally with ethanol and NNK without propranolol were found to have pancreatitis, and 66% developed pancreatic ductal adenocarcinoma [33]. Only one of 12 developed pancreatic ductal adenocarcinomas in animals treated with ethanol, NNK and propranolol. Animals treated with only ethanol had developed pancreatitis but no pancreatic cancer. Moreover, utilizing prior evidence of α7 nicotinic acetylcholine receptor (α7nAChR) activity in stimulation of the synthesis and release of adrenaline and noradrenaline to activate the cAMP pathway downstream of β-adrenergic receptors [32-34], the authors analyzed activity in all the three groups. Western blotting analysis performed of pancreatic cells harvested found 1.9-fold increase in α7nAChR protein in pancreatic ductal adenocarcinoma of those animals treated without propranolol. The one animal with pancreatic ductal adenocarcinoma that was treated with propranolol was found to have receptor activity to be lower than the control group.
A meta-analysis on 319,006 patients was performed by Na et al in 2018 to look at the effects of β-blockade on the prognosis of malignancies [35]. They found β-blocker use was associated with improved overall survival among patients with pancreatic cancer, as well as ovarian cancer (hazard ratio (HR) = 0.59, 95% confidence interval (CI): 0.36 - 0.96, P = 0.034) and melanomas (HR = 0.81, 95% CI: 0.67 - 0.97, P = 0.026). In the meta-analysis, two studies were included which involved the use of β-blockers in 16,092 patients with pancreatic cancer and found prolonged overall survival for patients with time-fixed post-diagnostic β-blocker use with HR of 0.85 (95% CI: 0.75 - 0.97, P = 0.014) [35]. Despite significant prolongation of overall survival shown, no statistically significant prolongation in overall survival was seen when analyzing use of selective (HR = 0.93, 95% CI: 0.83 - 1.05, P = 0.243) and non-selective β-blocker therapies (HR = 0.93, 95% CI: 0.83 - 1.05, P = 0.243). As such, definitive conclusions regarding the overall survival of patients with the use of specific class or formulations of β-blockers remain to be seen.
| Conclusion | ▴Top |
Association with β-blocker administration and the overall cancer prognosis in patients with pancreatic adenocarcinoma does show some promise. With this review we hope to provide current information for internists and specialists alike. With its use showing promising results in multiple experiments and patient meta-analysis, it remains to be seen whether its use will occur as part of guidelines as a chemo-preventive agent. Further studies need to be performed to assess relationships different classes and formulations of β-blockers have on the overall survival of patients with pancreatic adenocarcinoma. We need more high-quality studies, such as retrospective and prospective cohort studies, to establish a conclusion for the future.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Author Contributions
VU, SD, BG, JG and NU were responsible for writing various aspects of this manuscript. VU, SD, QN and SS were responsible for editing of this manuscript. JW was responsible for the creation of the figure included in this manuscript. AA, QN and SS were responsible for the final approval of this manuscript for submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
β2AR: β2-adrenergic receptor; GPCR: G-protein-coupled receptor; AC: adenylate cyclase; cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; MAPK: mitogen-activated protein kinase; C/EBPα: CCAAT enhancer binding protein; PDAC: pancreatic ductal adenocarcinoma cell; CREB: cAMP response element binding protein; AP-1: activator protein 1; EGFR: epidermal growth factor receptor; MMP: matrix metalloproteinase; VEGF: vascular endothelial growth factor; GR: glucocorticoid receptor; α7nAChR: α7 nicotinic acetylcholine receptor
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
doi pubmed - Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Wang YT, Gou YW, Jin WW, Xiao M, Fang HY. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212.
doi pubmed - Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970-2009. J Natl Cancer Inst. 2013;105(22):1694-1700.
doi pubmed - Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44(1):186-198.
doi pubmed - Peixoto R, Pereira ML, Oliveira M. Β-blockers and cancer: where are we? Pharmaceuticals (Basel). 2020;13(6):105.
doi pubmed - Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs. 2009;20(6):477-482.
doi pubmed - Montoya A, Varela-Ramirez A, Dickerson E, Pasquier E, Torabi A, Aguilera R, Nahleh Z, et al. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J. 2019;42(3):155-165.
doi pubmed - Phadke S, Clamon G. Beta blockade as adjunctive breast cancer therapy: A review. Crit Rev Oncol Hematol. 2019;138:173-177.
doi pubmed - Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628-638.
doi pubmed - Choy C, Raytis JL, Smith DD, Duenas M, Neman J, Jandial R, Lew MW. Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative beta-blockade. Oncol Rep. 2016;35(6):3135-3142.
doi pubmed - Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, Zhang M. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22(4):825-830.
doi - Zhang D, Ma Q, Shen S, Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist's anticancer effect in pancreatic cancer cell. Pancreas. 2009;38(1):94-100.
doi pubmed - Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22(3):473-479.
doi pubmed - Zhang D, Ma Q, Wang Z, Zhang M, Guo K, Wang F, Wu E. beta2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathway. Mol Cancer. 2011;10:146.
doi pubmed - Udumyan R, Montgomery S, Fang F, Almroth H, Valdimarsdottir U, Ekbom A, Smedby KE, et al. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. 2017;77(13):3700-3707.
doi pubmed - Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356-363.
doi pubmed - Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(Suppl 2):1-15.
doi pubmed - Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol. 2006;117(1):18-24; quiz 25.
doi pubmed - Philipson LH. beta-Agonists and metabolism. J Allergy Clin Immunol. 2002;110(6 Suppl):S313-317.
doi pubmed - Partecke LI, Speerforck S, Kading A, Seubert F, Kuhn S, Lorenz E, Schwandke S, et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology. 2016;16(3):423-433.
doi pubmed - Chang A, Kim-Fuchs C, Le CP, Hollande F, Sloan EK. Neural regulation of pancreatic cancer: a novel target for intervention. Cancers (Basel). 2015;7(3):1292-1312.
doi pubmed - Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115-1128.
doi pubmed - Huang XY, Wang HC, Yuan Z, Huang J, Zheng Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology. 2012;59(115):889-893.
doi pubmed - Wang L, Bai YY, Yang Y, Hu F, Wang Y, Yu Z, Cheng Z, et al. Diabetes mellitus stimulates pancreatic cancer growth and epithelial-mesenchymal transition-mediated metastasis via a p38 MAPK pathway. Oncotarget. 2016;7(25):38539-38550.
doi pubmed - Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P. Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol. 2004;49(3):179-186.
doi pubmed - Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357-10364.
doi pubmed - Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell. 2018;33(1):75-90 e77.
doi pubmed - Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21(2):139-146.
doi pubmed - Hong SH, Avis I, Vos MD, Martinez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer Res. 1999;59(9):2223-2228.
- Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221(3):261-267.
doi pubmed - Al-Wadei HA, Schuller HM. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. 2009;218(4):437-445.
doi pubmed - Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69(2):618-628.
doi pubmed - Na Z, Qiao X, Hao X, Fan L, Xiao Y, Shao Y, Sun M, et al. The effects of beta-blocker use on cancer prognosis: a meta-analysis based on 319,006 patients. Onco Targets Ther. 2018;11:4913-4944.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


