| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Short Communication
Volume 12, Number 10, October 2020, pages 674-680
Low-Flow Nasal Cannula Hydrogen Therapy
Motoaki Sanoa, b, d, Kohsuke Shirakawaa, b, Yoshinori Katsumataa, b, Genki Ichiharaa, b, Eiji Kobayashia, b, c
aDepartment of Cardiology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
bCenter for Molecular Hydrogen Medicine, Keio University, 2-15-45 Mita, Minato-ku, Tokyo 108-8345, Japan
cDepartment of Organ Fabrication, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
dCorresponding Author: Motoaki Sano, Department of Cardiology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
Manuscript submitted August 12, 2020, accepted August 21, 2020, published online September 21, 2020
Short title: Low-Flow Hydrogen Therapy
doi: https://doi.org/10.14740/jocmr4323
| Abstract | ▴Top |
Background: Molecular hydrogen (H2) is a biologically active gas that is widely used in the healthcare sector. In recent years, on-site H2 gas generators, which produce high-purity H2 by water electrolysis, have begun to be introduced in hospitals, clinics, beauty salons, and fitness clubs because of their ease of use. In general, these generators produce H2 at a low-flow rate, so physicians are concerned that an effective blood concentration of H2 may not be ensured when the gas is delivered through a nasal cannula. Therefore, this study aimed to evaluate blood concentrations of H2 delivered from an H2 gas generator via a nasal cannula.
Methods: We administered 100% H2, produced by an H2 gas generator, at a low-flow rate of 250 mL/min via a nasal cannula to three spontaneously breathing micro miniature pigs. An oxygen mask was placed over the nasal cannula to administer oxygen while minimizing H2 leakage, and a catheter was inserted into the carotid artery to monitor the arterial blood H2 concentration.
Results: During the first hour of H2 inhalation, the mean (standard error (SE)) H2 concentrations and saturations in the arterial blood of the three pigs were 1,560 (413) nL/mL and 8.85% (2.34%); 1,190 (102) nL/mL and 6.74% (0.58%); and 1,740 (181) nL/mL and 9.88% (1.03%), respectively. These values are comparable to the concentration one would expect if 100% of the H2 released from the H2 gas generator is taken up by the body.
Conclusions: Inhalation of 100% H2 produced by an H2 gas generator, even at low-flow rates, can increase blood H2 concentrations to levels that previous non-clinical and clinical studies demonstrated to be therapeutically effective. The combination of a nasal cannula and an oxygen mask is a convenient way to reduce H2 leakage while maintaining oxygenation.
Keywords: Hydrogen gas; Pharmacokinetics; Hydrogen gas inhaler; Hydrogen gas generator; Micro miniature pig; Combined oxygen masks with nasal cannula; COVID-19
| Introduction | ▴Top |
Molecular hydrogen (H2) has a wide range of benefits, ranging from improving health to preventing and treating disease. The health benefits include improving mood, reducing anxiety, and counteracting an overactive sympathetic nervous system [1]. In terms of disease prevention and treatment, H2 has been used to treat many diseases, including lifestyle diseases [2]; immune diseases, such as atopic dermatitis [2], hay fever [3], and chronic rheumatoid arthritis [4]; respiratory diseases, such as asthma [5], chronic obstructive pulmonary disease [6], and pneumonia caused by the coronavirus disease 2019 (COVID-19) [7]; neurological diseases, such as depression [8], dementia [9], stroke [10], post-cardiac arrest syndrome [11, 12], subarachnoid hemorrhage [13], and traumatic injury from blast shock waves [14]; myocardial infarction [15-17]; chronic kidney disease [18]; sepsis [19] and hemorrhagic shock [20, 21]; and cancer [22].
H2 exerts antioxidant and anti-inflammatory effects. As a molecular mechanism, ex vivo experiments have been shown that H2 reduces hydroxyl radical (·OH) and peroxynitrite [10] and suppresses the propagation of the radical reaction in lipid bilayers [23]. Clinical and animal studies have shown that H2 therapy reduces circulating levels of oxidative stress markers and proinflammatory cytokines in shock from a wide range of etiologies [11, 24].
H2 can be supplied from a high-pressure gas cylinder [11, 12, 15-17], generated from a hydrogen-absorbing alloy [25, 26] or hydride, or produced by electrolysis of water [7]. In recent years, on-site H2 gas generators, which generate high-purity H2 by electrolysis of water, have begun to be introduced in hospitals, clinics, beauty salons, and fitness clubs because of their ease of handling.
Recently, the results of a clinical study on the therapeutic effect of H2 inhalation in COVID-19 pneumonia were reported from China [7]. In this study, the researchers used an H2 gas generator as the source of H2 and administered a mixture of H2 and oxygen (O2) gas (66% H2; 33% O2), obtained by electrolysis of water, by a nasal cannula. An improvement in clinical symptoms was seen in a significantly higher percentage of patients in the treatment group, who inhaled a mixture of H2 and O2, than in patients in the control group, who received classic oxygen therapy.
Because the H2 produced from an on-site H2 gas generator has a low-flow rate, clinicians are concerned that when H2 is inhaled through a nasal cannula, blood concentrations may be lower than expected because of leakage of H2 from the nostrils. We hypothesized that the H2 concentration could be better ensured if patients wore an oxygen mask over the nasal cannula. Therefore, we tested this hypothesis in pigs, whose organs are similar in size, anatomy, and physiology to those of humans.
| Materials and Methods | ▴Top |
Animals
The present study was designed according to the principles of the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines [27]. The experiments were performed in accordance with our institutional guidelines and with the Japanese law on the protection and management of animals. Ethical approval was granted by the Research Council and Animal Care and Use Committee of Keio University (approval no: 12094-(8)).
The study was performed in three micro miniature pigs, weighing 16.8 kg, 15.5 kg, and 16.8 kg, which were housed in separate cages under temperature- and light-controlled conditions (12-h light/dark cycle) and provided with food and water ad libitum (Supplementary Material 1, www.jocmr.org). Before surgery, the pigs were fasted for 12 h, with free access to water. Then, a xylazine hydrochloride intramuscular injection was given 15 min before induction of isoflurane anesthesia. Experiments were performed under anesthesia, and isoflurane was administered to maintain immobilization.
Catheter insertion
First, a central venous catheter (14 gauge × 70 cm; Argyle) equipped with a three-way stopcock (TERUMO terufusion three-way stopcock, R type) was filled with heparinized saline. Once at a sufficient depth of anesthesia, each pig was placed in the supine position. A vertical incision of about 10 cm was made in the right side of the neck to expose about 3 cm of the right external jugular vein and the right internal carotid artery. The peripheral side of the right internal carotid artery was ligated with a 1-0 silk thread, a bulldog clip was applied to the medial side, an incision was made, and a catheter was advanced about 5 cm into the artery and secured. Surgery was performed by the senior author of this paper (EK), a surgeon who has completed more than 200 clinical transplant operations and is a steering member of the transplantation society and a permanent director of the transplantation society of Japan. Blood was collected from the intravascular catheters at 0, 10, 30, and 60 min after starting H2 inhalation, and the blood H2 concentration was measured by gas chromatography.
H2 gas generator
This study used the H2 inhaler H2JI1, manufactured by Doctors Man Co, Ltd. The inhaler uses a proton-exchange membrane (PEM) water electrolysis system that can continuously generate high purity (> 99.999%) H2 at a flow rate of 250 mL/min, 24 h a day, 365 days a year (Supplementary Material 2, www.jocmr.org).
H2 inhalation
The length of the part of the nasal cannula (Nakamura Medical Industry Co., Ltd.) that is inserted into the nostrils was modified from the original 12 mm to 62 mm by inserting a 10-mm silicon tube with a total length of 60 mm, an outer diameter of 3 mm, and an inner diameter of 1.5 mm into the left and right outlets. We then adjusted the nostril inserts of the nasal cannula to fit the shape of a pig’s nose. The nasal cannula was inserted deep into the nasal cavity of a pig under spontaneous breathing, and 100% H2 produced from the H2 gas generator was administered at a flow rate of 250 mL/min (Fig. 1).
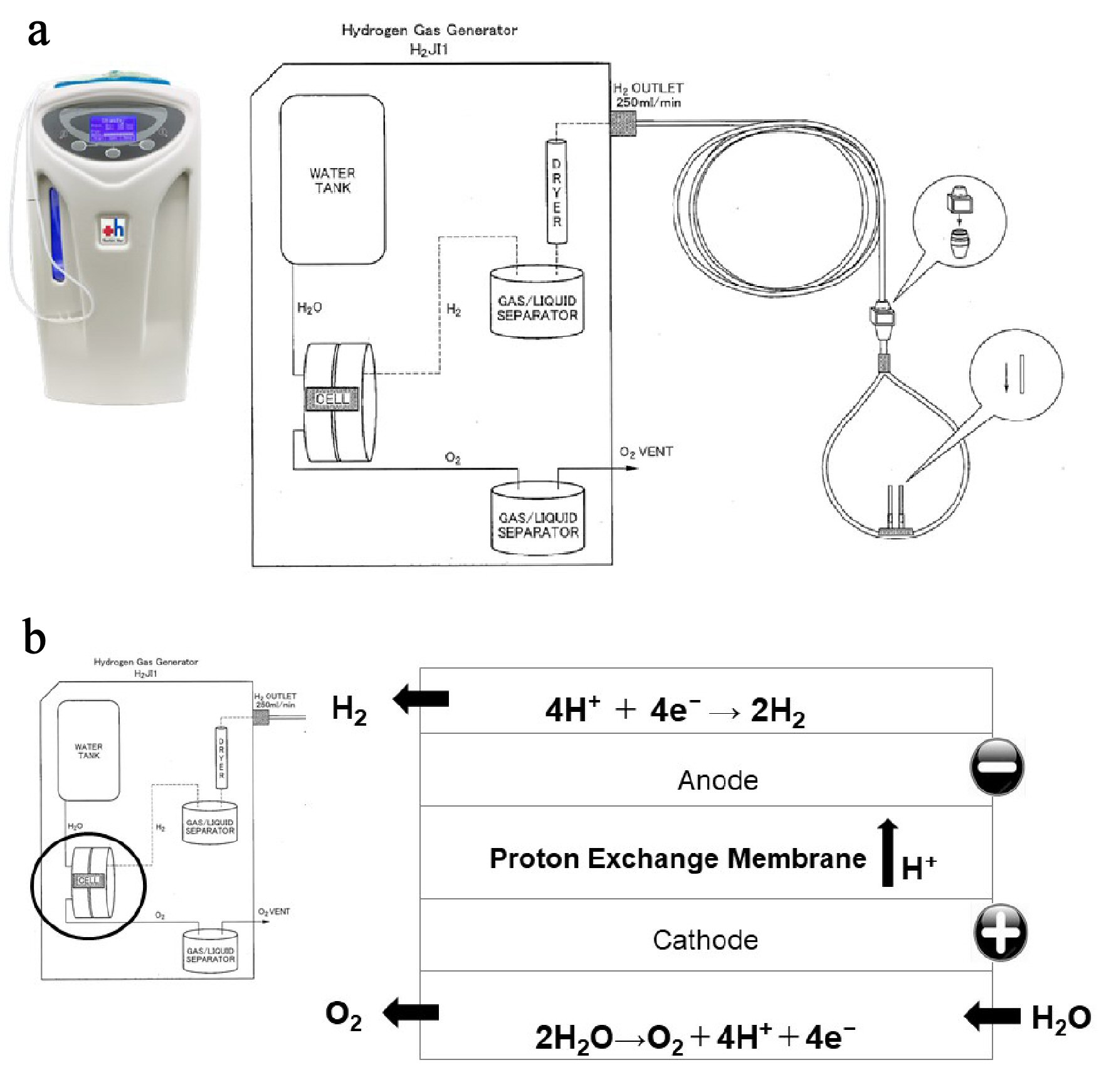 Click for large image | Figure 1. Hydrogen gas (H2) supply system from an H2 gas generator. (a) Specifications of H2 gas generator. The H2 gas generator is capable of continuously administering 100% H2 at a flow rate of 250 mL/min, 24 h a day, 365 days a year. (b) Structure of the electrolyzer. The electrolysis reaction occurs in an electrolyzer, which consists of two electrodes separated by an ion exchange membrane. When voltage is continuously applied to the electrodes in the electrolyzer, two electrons are removed from a water molecule at the anode (negative electrode) to form one oxygen molecule (O2) and four hydrogen ions. The O2 is safely released into the atmosphere, and the four hydrogen ions pass through the ion exchange membrane and are attracted to the cathode. At the cathode (positive electrode), electrons are combined with hydrogen ions to produce hydrogen gas (H2). |
A veterinary anesthesia mask (Shinano Manufacturing Co., Ltd.) was placed over the nasal cannula and an ADS 1000 (model: 2000) veterinary anesthesia delivery system (Tokushima Iryoki Co., Ltd.) was used to deliver a mixture of O2 and isoflurane (Fig. 2). The flow rate of the O2/isoflurane gas mixture was maintained above 6 L/min to avoid re-inhalation of exhaled carbon dioxide (CO2) that would stay in the mask.
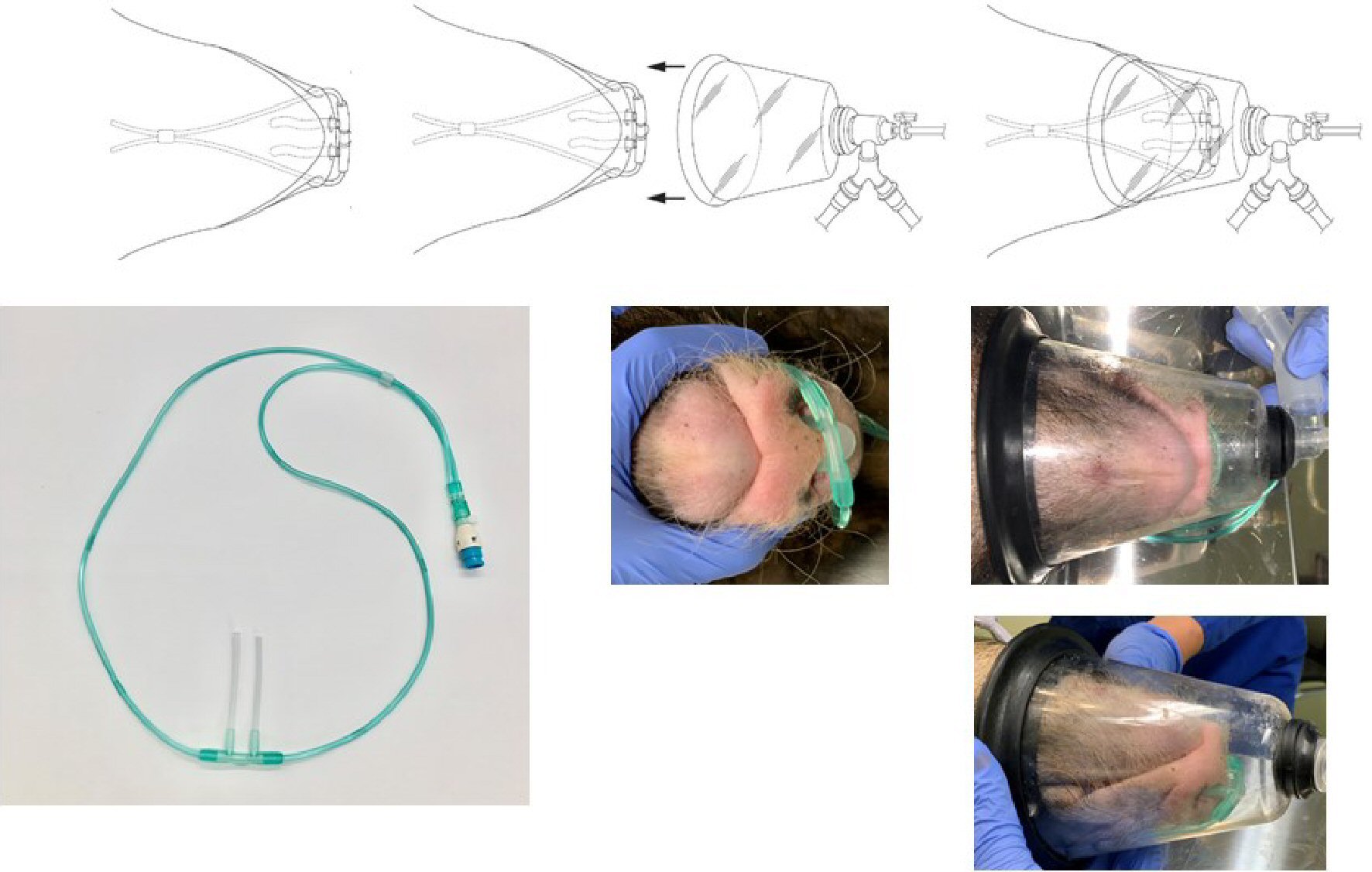 Click for large image | Figure 2. Nasal cannula and oxygen mask for micro miniature pigs. The length of the part of the nasal cannula that is inserted into the nostrils was modified from the original 12 mm to 62 mm by inserting a 10-mm silicon tube. The nasal cannula was then inserted deep into the nasal cavity of a spontaneously breathing pig, and 100% H2 produced by the H2 gas generator was administered at a flow rate of 250 mL/min. A veterinary anesthesia mask was placed over the nasal cannula, and a veterinary anesthesia delivery system was used to supply oxygen and anesthesia to the animals. |
Measurement of H2 concentration
To measure the blood H2 concentration, we first inserted a needle into the rubber lid of a 13.5-mL sealed vial, extracted 1 mL of air and injected 1 mL of blood. To prevent outgassing, we immediately applied wax to the rubber lid to seal the injection hole. H2 in the blood was released into the air phase in the closed vial. Some of the air phase (0.2 mL, 0.4 mL, or 1 mL, depending on the H2 concentration) was collected from the vial, and the H2 concentration was measured by gas chromatography (TRIlyzer mBA-3000, Taiyo, Co., Ltd.). A calibration curve was obtained by using standard H2 gas of 0, 5, 50, and 130 ppm. Each sample was measured twice. The concentration of the sample taken before H2 inhalation was subtracted as the background [26].
| Results | ▴Top |
In all three pigs, the blood H2 concentrations in the carotid arteries before inhalation were almost 0, reached peak levels by 10 min after the start of inhalation and remained at approximately the same level until 60 min later. The means (standard error (SE)) of the H2 concentrations and saturations in the blood from the carotid arteries during H2 inhalation in the three pigs were 1,560 (413) nL/mL and 8.85% (2.34%); 1,190 (102) nL/mL and 6.74% (0.58%); and 1,740 (181 nL/mL) and 9.88% (1.03%), respectively (Fig. 3 and Supplementary Material 3, www.jocmr.org).
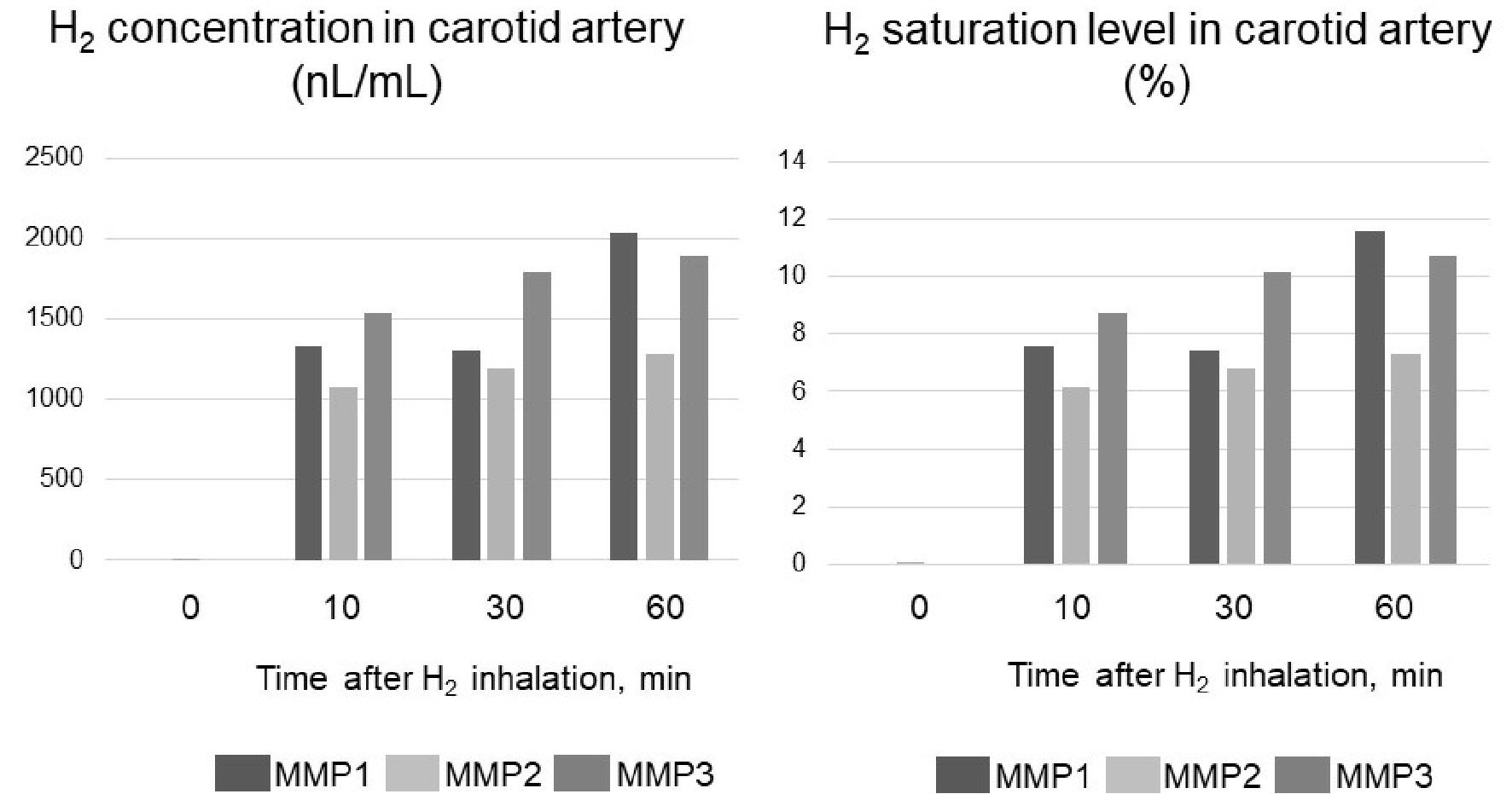 Click for large image | Figure 3. Carotid artery hydrogen (H2) concentration in three micro miniature pigs (MMP1 - 3) during H2 inhalation. The concentration of H2 when it is completely dissolved in water is 17,600 nL/mL. Therefore, H2 saturation was determined by dividing the measured value (concentration) by 17,600 nL/mL. |
We calculated the fraction of inspiratory H2 from the H2 flow rate produced by the H2 gas generator, as follows: The H2 flow rate per second was 250/60 mL, so at a respiratory rate of 20 breaths per minute, the expected volume of H2 that would accumulate in the nasopharynx during 3-s inhalation time and exhalation was 250/60 mL × 3 = 12.5 mL. If H2 was inhaled without leakage from the nostrils, the fraction of inspiratory H2 would be 12.5/150 = 0.083, or 8.3% at a tidal volume of 150 mL. In short, the measured H2 concentration (saturation) in the arterial blood agreed closely with the expected value predicted from the H2 gas generator’s flow rate.
| Discussion | ▴Top |
In this experiment to establish a methodology for efficiently delivering 100% H2 with an on-site H2 gas generator, we administered H2 to three pigs through a nasal cannula with an oxygen mask worn over it. Our study showed that an effective blood H2 concentration can be achieved even with the low-flow rates of the H2 gas generator.
The H2JI1 H2 gas generator used in this study consistently supplies 100% H2 with a purity greater than 99.999% at a flow rate of 250 mL/min. If such a generator is used to administer H2 to a human, at a tidal volume of 500 mL and 20 breaths per minute the expected inspired H2 concentration would be 2.5%. This concentration would be even higher in the elderly, who have a lower tidal volume.
In our previous clinical study to evaluate the safety and efficacy of H2 inhalation in patients with ST-segment elevation myocardial infarction [17], we delivered H2 via high-pressure compressed gas cylinders containing a mixture of H2 (1.3%), O2, nitrogen, and mixed gases through a mask at a high-flow rate of 10 L/min. We chose this flow rate to ensure that the concentration of inhaled H2 was 1.3% because this concentration was confirmed to be effective in reducing infarct size in preclinical studies in dogs [16]. The High-Pressure Gas Safety Act stipulates that when O2 and H2 are mixed under pressure, the H2 concentration must be no greater than 1.3%. In the present study, we found that even though the flow of 100% H2 generated on site by the H2 gas generator was low, if H2 was administered by a nasal cannula covered with an oxygen mask the arterial blood H2 saturation levels increased to the same levels as when high-flow 1.3% H2 was administered from compressed gas high-pressure cylinders.
The H2 gas generator is lightweight and portable and can provide H2 anywhere, as long as a power source and pure water are available. Furthermore, the generator can supply H2 continuously for a long time. The generator represents a safe and convenient alternative to high-pressure gas cylinders for H2 inhalation therapy and requires no replenishment of supplies. For example, installing an H2 gas generator at the bedside of a patient with COVID-19 pneumonia with hypoxemia allows the patient to consistently inhale H2 through a nasal cannula. In addition, a suitable mask (face mask, face mask with reservoir, or Venturi mask) can be worn over the nasal cannula, depending on the patient’s level of oxygenation. Using this method, a patient can continuously inhale H2 until the pneumonia is cured and the patient is discharged from the hospital. Similarly, patients with mild cases of COVID-19 who are waiting outside a hospital can be given H2 inhalation to prevent their illness becoming more severe.
Although some people experience a headache after inhaling H2, probably due to the dilatation of intracranial blood vessels, no other obvious symptoms are known that could be considered adverse events. Unlike the other bioactive gases, such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), H2 does not bind to the heme in the hemoglobin in the red blood cells. Therefore, the O2 saturation and the partial pressures of O2 and CO2 in arterial blood are unaffected by the inhalation of H2 under steady-state conditions [15].
H2 is the most abundant element in the universe, but it must be produced because it does not occur naturally in a gaseous state on Earth. H2 has been used in laboratories for a variety of applications, including gas chromatography and inductively coupled plasma-mass spectrometry. In addition, it is used in the chemical industry to synthesize ammonia, cyclohexane, and methanol, and in the food industry to hydrogenate oils to make them more solid. Recently, it has been gaining attention as a clean energy source. The best way to produce high-purity H2 on demand is by electrolysis of water. A noteworthy innovation in this technology was water electrolysis with solid polymer electrolyte membranes proposed by General Electric in the early 1970s. Subsequent significant research and development led to a technology for efficiently and stably generating high-purity H2 from pure water 24 h a day, 365 days a year. This method for generating H2, which has been cultivated by the industry for a long time, has found many uses in humans. The H2 gas generator H2JI1 that we used in our experiments also uses a polymer electrolyte membrane with a durability of more than 50,000 h. The amount of H2 that remains in this device is small, so safety is guaranteed. Also, the device is not subject to the High-Pressure Gas Safety Act. Although the hydrogen gas generator itself is expensive, the durability of the electrolytic cells is long and the running costs are almost zero. Therefore, the cost per hour is as low as less than $0.47.
Of course, both high-pressure gas cylinders and H2 gas generators must be used appropriately, depending on a patient’s situation. When administering H2 via a ventilator, physicians should choose high-pressure gas cylinders capable of delivering high-flow rates of gas. However, if physicians wish to administer H2 continuously over a long period of time to patients who are breathing spontaneously, we propose that they should consider using a H2 gas generator that can safely produce low-flow but 100% H2 on-site.
Not much time has passed since O2 inhalation began to be used in medical settings. First, O2 was widely used in industries such as welding and cutting because it supports combustion, but during 1918 - 1920 physicians starting using O2 to treat the “Spanish flu.” Citizens rushed to industrial O2 supply companies and queued all night waiting for O2 to arrive. In light of the current COVID-19 pandemic, we propose that now is the time to reconsider the benefits of H2 inhalation therapy.
| Supplementary Material | ▴Top |
Suppl 1. Overview of the Micro Miniature Pigs Used in the Experiments.
Suppl 2. Specifications of the Hydrogen Gas Generator H2JI1.
Suppl 3. Mean Hydrogen (H2) Concentration in the Carotid Artery of Three Micro Miniature pigs (MMP1 - 3).
Acknowledgments
The authors are grateful to Suga Kato ((Japanese Molecular Hydrogen Promotion Association (JHyPA)) and Mayumi Takeda (JHyPA) for technical assistance.
Financial Disclosure
This work was supported by grants from Doctors Man Co., Ltd. The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Conflict of Interest
MS and EK receive advisory fees and research fees from Doctors Man Co., Ltd. MS receives advisory fees and research fees from Taiyo Nippon Sanso. The authors would like to declare the following patents/patent applications associated with this research: Author MS is the registered inventor of the following patents jointly filed by Keio University and Taiyo Nippon Sanso: hydrogen mixed gas supply device for medical purposes (patent number: 5631524), medicinal composition for improving prognosis after restart of patient’s own heartbeat, and medicinal composition for improving and/or stabilizing circulatory dynamics after onset of hemorrhagic shock. In addition to these, there are three other patents in which the name of the inventions are only in Japanese and not described in English. Here are the names of the inventions, which are literal translation of Japanese into English: pharmaceutical compositions for reducing weight loss after organ harvesting (Joint application with Keio University and Taiyo Nippon Sanso), method for generating organ preservation solution containing hydrogen and organ preservation solution containing hydrogen (Joint application with Keio University and Doctors Man; Application number PCT/JP2019/045790). This does not alter our adherence to Journal of Clinical Medicine Research policies on sharing data and materials.
Informed Consent
Not applicable.
Author Contributions
MS designed the study, oversaw data collection, reviewed the literature, analyzed and interpreted the data, and drafted the manuscript. EK contributed to the design of the study, engaged in data collection and provided critical reviews of the manuscript. KS, YK, and GI contributed to data collection and provided critical reviews of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Mizuno K, Sasaki AT, Ebisu K, Tajima K, Kajimoto O, Nojima J, Kuratsune H, et al. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med Gas Res. 2017;7(4):247-255.
doi pubmed - Yoon YS, Sajo ME, Ignacio RM, Kim SK, Kim CS, Lee KJ. Positive Effects of hydrogen water on 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Biol Pharm Bull. 2014;37(9):1480-1485.
doi pubmed - Fang S, Li X, Wei X, Zhang Y, Ma Z, Wei Y, Wang W. Beneficial effects of hydrogen gas inhalation on a murine model of allergic rhinitis. Exp Ther Med. 2018;16(6):5178-5184.
doi pubmed - Ishibashi T, Sato B, Rikitake M, Seo T, Kurokawa R, Hara Y, Naritomi Y, et al. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med Gas Res. 2012;2(1):27.
doi pubmed - Zhang N, Deng C, Zhang X, Zhang J, Bai C. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract. 2018;4:3.
doi pubmed - Lu W, Li D, Hu J, Mei H, Shu J, Long Z, Yuan L, et al. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J Thorac Dis. 2018;10(6):3232-3243.
doi pubmed - Guan WJ, Wei CH, Chen AL, Sun XC, Guo GY, Zou X, Shi JD, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. 2020;12(6):3448-3452.
doi pubmed - Zhang Y, Su WJ, Chen Y, Wu TY, Gong H, Shen XL, Wang YX, et al. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci Rep. 2016;6:23742.
doi pubmed - Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer's disease by reduction of oxidative stress. Brain Res. 2010;1328:152-161.
doi pubmed - Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688-694.
doi pubmed - Hayashida K, Sano M, Kamimura N, Yokota T, Suzuki M, Maekawa Y, Kawamura A, et al. H(2) gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J Am Heart Assoc. 2012;1(5):e003459.
doi pubmed - Hayashida K, Sano M, Kamimura N, Yokota T, Suzuki M, Ohta S, Fukuda K, et al. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation. 2014;130(24):2173-2180.
doi pubmed - Kumagai K, Toyooka T, Takeuchi S, Otani N, Wada K, Tomiyama A, Mori K. Hydrogen gas inhalation improves delayed brain injury by alleviating early brain injury after experimental subarachnoid hemorrhage. Sci Rep. 2020;10(1):12319.
doi pubmed - Satoh Y, Araki Y, Kashitani M, Nishii K, Kobayashi Y, Fujita M, Suzuki S, et al. Molecular hydrogen prevents social deficits and depression-like behaviors induced by low-intensity blast in mice. J Neuropathol Exp Neurol. 2018;77(9):827-836.
doi pubmed - Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373(1):30-35.
doi pubmed - Yoshida A, Asanuma H, Sasaki H, Sanada S, Yamazaki S, Asano Y, Shinozaki Y, et al. H(2) mediates cardioprotection via involvements of K(ATP) channels and permeability transition pores of mitochondria in dogs. Cardiovasc Drugs Ther. 2012;26(3):217-226.
doi pubmed - Katsumata Y, Sano F, Abe T, Tamura T, Fujisawa T, Shiraishi Y, Kohsaka S, et al. The Effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infarction- first pilot study in humans. Circ J. 2017;81(7):940-947.
doi pubmed - Chen J, Zhang H, Hu J, Gu Y, Shen Z, Xu L, Jia X, et al. Hydrogen-rich saline alleviates kidney fibrosis following AKI and retains klotho expression. Front Pharmacol. 2017;8:499.
doi pubmed - Qiu P, Liu Y, Zhang J. Recent advances in studies of molecular hydrogen against sepsis. Int J Biol Sci. 2019;15(6):1261-1275.
doi pubmed - Matsuoka T, Suzuki M, Sano M, Hayashida K, Tamura T, Homma K, Fukuda K, et al. Hydrogen gas inhalation inhibits progression to the "irreversible" stage of shock after severe hemorrhage in rats. J Trauma Acute Care Surg. 2017;83(3):469-475.
doi pubmed - Tamura T, Sano M, Matsuoka T, Yoshizawa J, Yamamoto R, Katsumata Y, Endo J, et al. Hydrogen gas inhalation attenuates endothelial glycocalyx damage and stabilizes hemodynamics in a rat hemorrhagic shock model. Shock. 2020;54(3):377-385.
doi pubmed - Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X, Zhou J, et al. Hydrogen gas in cancer treatment. Front Oncol. 2019;9:696.
doi pubmed - Iuchi K, Imoto A, Kamimura N, Nishimaki K, Ichimiya H, Yokota T, Ohta S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016;6:18971.
doi pubmed - Tamura T, Suzuki M, Hayashida K, Kobayashi Y, Yoshizawa J, Shibusawa T, Sano M, et al. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J Clin Biochem Nutr. 2020; Epub ahead of print.
doi pubmed - Kobayashi E, Sano M. Organ preservation solution containing dissolved hydrogen gas from a hydrogen-absorbing alloy canister improves function of transplanted ischemic kidneys in miniature pigs. PLoS One. 2019;14(10):e0222863.
doi pubmed - Sano M, Ichihara G, Katsumata Y, Hiraide T, Hirai A, Momoi M, Tamura T, et al. Pharmacokinetics of a single inhalation of hydrogen gas in pigs. PLoS One. 2020;15(6):e0234626.
doi pubmed - Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


