| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 10, October 2019, pages 696-702
Is Bedside End-Tidal CO2 Measurement a Screening Tool to Exclude Pulmonary Embolism in Emergency Department?
Metin Ozdemira, e, Bedriye Muge Sonmezb, Fevzi Yilmazb, Aykut Yilmazc, Murat Duyanb, Seval Komutd
aDepartment of Emergency Medicine, Istanbul Esenyurt Necmi Kadioglu State Hospital, Istanbul, Turkey
bDepartment of Emergency Medicine, Antalya Education and Research Hospital, University of Health Sciences, Antalya, Turkey
cDepartment of Cardiology, Siirt State Hospital, Siirt, Turkey
dDepartment of Emergency Medicine, Erol Olcok Education and Research Hospital, Hitit University, Corum, Turkey
eCorresponding Author: Metin Ozdemir, Department of Emergency Medicine, Istanbul Esenyurt Necmi Kadioglu State Hospital, Fatih Mh. 19 Mayis Bulvari No.8, Istanbul 34510, Turkey
Manuscript submitted August 1, 2019, accepted August 24, 2019
Short title: A Challenging Diagnosis With ETCO2
doi: https://doi.org/10.14740/jocmr3941
| Abstract | ▴Top |
Background: Pulmonary embolism (PE) is among the most difficult conditions to diagnose in emergency department. The majority of patients thought to have PE are tested positive for D-dimer and subsequently tested with advanced diagnostic modalities. Novel noninvasive tests capable of excluding PE may obviate the need for advanced imaging tests. We studied the role of combined clinical probability assessment and end-tidal carbon dioxide (ETCO2) measurement for diagnosis of possible PE in emergency department.
Methods: We included 100 consecutive subjects suspected to have PE and a positive D-dimer test to study clinical probability of PE and ETCO2 levels. ETCO2 > 34 mm Hg was found to be the best cut-off point for diagnosing PE. PE was ultimately eliminated or diagnosed by spiral computed tomography (CT).
Results: Diagnostic performances of tests were as follows: ETCO2 and D-dimer had a sensitivity of 100% and a negative predictive value (NPV) of 100% at the cut-off levels of 34 mm Hg and 500 ng/mL, respectively; Wells score had a sensitivity of 80% and NPV of 69.7% at a score of 4.
Conclusions: ETCO2 alone cannot reliably exclude PE. Combining it with clinical probability, however, reliably and correctly eliminates or diagnoses PE and prevents further testing to be done.
Keywords: Pulmonary embolism; End-tidal carbon dioxide; Emergency department
| Introduction | ▴Top |
Pulmonary embolism (PE) is a common condition that is difficult to diagnose. It has been attempted to obviate the need for the gold standard test, computed tomography pulmonary angiography (CTPA), to diagnose PE. Serving that purpose, D-dimer is a frequently performed test that reliably excludes PE [1]. Unfortunately, it possesses a low specificity in the elderly, pregnant subjects, and cancer patients, reducing the negative predictive power to 30% [2]. Thus, in the ED additional noninvasive bedside tests are needed to reduce costs and morbidity of diagnostic tests for PE.
PE is characterized by lung portions that are adequately ventilated but poorly perfused, which results in a lower amount of CO2 released into air and creation of an alveolar dead space [3]. Whereas quantification of dead space and arterial-alveolar CO2 tension gradient have been tested in patients with PE, these measurements lack a decent sensitivity to eliminate the possibility of PE in a reliable manner [4, 5]. End-tidal carbon dioxide (ETCO2), a bedside test, shows vascular occlusion in PE and can reduce side effects of diagnostic tests. It is non-invasive, safe, and cheap. In contrast, dead space can be measured only by collecting exhaled gases and alveolar-arterial gradient necessitates making arterial blood gas analysis [6].
Herein, we aimed to combine clinical probability and end-tidal ETCO2 measurement in a prospective manner in order to show whether this approach may exclude or confirm suspected PE.
| Materials and Methods | ▴Top |
Study design
This study was approved by the scientific research assessment commission and prospectively performed at an emergency department (ED) of an urban training and research hospital, between January 15, 2015 and April 15, 2015. Informed consent form was obtained from the patients and patient relatives.
Patient population and eligibility criteria
A prospectively gathered convenience cohort of 100 subjects, 50 of whom presented to ED with various complaints and were confirmed to have PE with CTPA (study group) based on a high Wells score or a positive plasma D-dimer, and 50 of whom were confirmed to not have PE with CTPA (control group), were eligible for the study. Patients below 18 years, who did not provide a written consent or who refused to participate in the study, who had hypercapnic respiratory failure, neuromuscular disease, pregnancy malignant hyperthermia, bicarbonate infusion, non-invasive ventilation, and oxygen therapy > 4 L/min that could potentially affect ETCO2 level, were excluded from the study.
Patient assessment and data collection
Patients who presented to ED and had suspected PE were risk stratified as low, moderate, and high risk according to the Wells scoring system. All patients underwent ETCO2 measurement using a capnogrpahy (EMMATM Emergency Capnometer). ETCO2 measurements were carried out by placing the capnography device between the endotracheal tube and bag-valve mask (BVM) in intubated patients and by placing it to the tip of the BVM in the non-intubated patients. Patients were instructed to breathe normally and were tested for five breaths in either a supine or seated position. Nostrils were not clipped shut. ETCO2 for each breath and respiratory rate was measured.
Age, sex, comorbid conditions, admission symptoms, vital parameters (blood pressure, pulse rate, respiratory rate, body temperature, and oxygen saturation), ETCO2, blood gas analyses, D-dimer levels, Wells score [7], and mortality rates were recorded in study forms. Pulmonary CT was used to confirm or exclude pulmonary embolism.
Statistical analysis
Statistical analyses were performed with SPSS (Statistical Package for Social Sciences) Windows 19 software package. Normality of distribution of continuous and discrete numerical values was tested using the Kolmogorov-Smirnov test. Descriptive statistics included mean ± standard deviation for continuous numerical variable; median and interquartile range (IQR) for discrete numerical values; and number and percentage (%) for categoric variables. Continuous variables were compared using the Student’s t-test and Mann-Whitney U-test while the categorical variables were compared using the Chi-square test. Factors affecting pulmonary thromboembolism (PTE) positivity were assessed with logistic regression analysis. The results were presented at a confidence interval of 95% and a statistical significance level of P < 0.05.
| Results | ▴Top |
The patient group had a mean age of 66.3 ± 18.6 years; the control group had a mean age of 66.0 ± 14.9 years. The two groups were not significantly different with respect to age (P > 0.05). The patient group consisted of 32 (64%) women and 18 (36%) men; the control group consisted of 22 (44%) women and 28 (56%) men. The PE-positive group had a significantly higher percentage of women (P < 0.05). The most common comorbid condition in both groups was hypertension. The two groups were not significantly different with respect to the prevalence of comorbid conditions (P > 0.05).
The PE and non-PE groups had similar primary admission symptoms, state of consciousness, state of intubation, respiratory auscultation findings, blood pressure, pulse rate, respiratory rate, body temperature, and electrocardiograph (ECG) findings (P > 0.05). A comparison of arterial blood gas analysis revealed no significant inter-group differences for pH, pCO2, and HCO3 levels but pO2 level was significantly lower in the patient group (P < 0.05). The PE group had a significantly higher hospital admission rate than the non-PE group (P < 0.05) (Table 1).
 Click to view | Table 1. Clinical and Demographical Characteristics of Patients |
An analysis of the Wells scores showed that the median Wells score of the PE group was 6 (IQR: 2), and the non-PE group had a median Wells score of 4 (IQR: 1.25). Ten (20%) patients with PE were deemed high risk whereas no subject in the non-PE group was in the high-risk group. The predictive power of the Wells score for PE was significantly greater in the high-risk group (P < 0.001) (Fig. 1, Table 2).
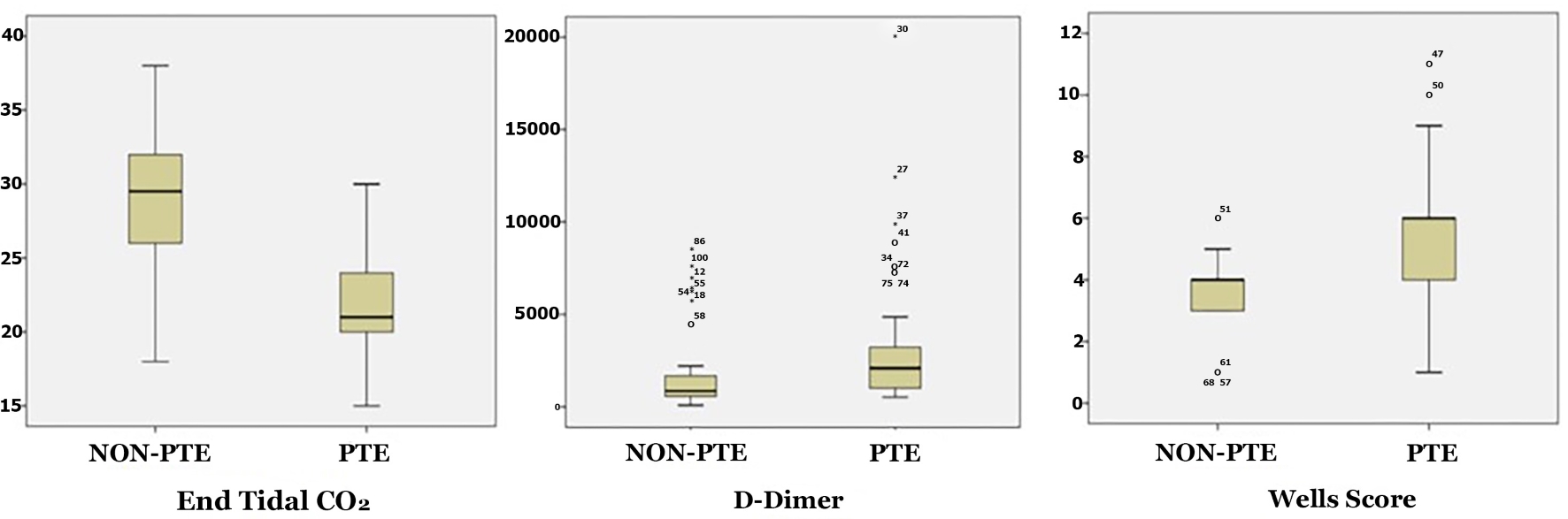 Click for large image | Figure 1. Comparison of ETCO2, D-dimer and Wells scores of the PE and non-PE groups. ETCO2: end-tidal carbon dioxide; PE: pulmonary embolism. |
 Click to view | Table 2. Assessment of the Wells Score |
The PE group had a median ETCO2 of 21 (IQR: 4) and the non-PE group 28.5 (IQR: 6). ETCO2 was significantly lower in the PE group (P < 0.001). The PE group had a median D-dimer of 2,091 ng/mL (IQR: 2,332 ng/mL) while the non-PE group had a median D-dimer level of 2,091 ng/mL (IQR: 2,332 ng/mL). The PE group had a significantly higher D-dimer level than the non-PE group (P < 0.001) (Fig. 1, Table 3).
 Click to view | Table 3. ETCO2 and D-Dimer Levels |
For diagnosing PE, a cut-off level of 34 mm Hg for ETCO2 had a sensitivity of 100%, a specificity of 8%, a positive predictive value (PPV) of 52.1% and a negative predictive value (NPV) of 100%. At a cut-off level of 500 ng/mL, D-dimer had a sensitivity of 100%, a specificity of 18%, a PPV of 54.9% and an NPV of 100%. Wells score, a cut-off level of 4, had a sensitivity of 80%, a specificity of 46%, a PPV of 59.7% and an NPV of 69.7% (Fig. 2, Table 4).
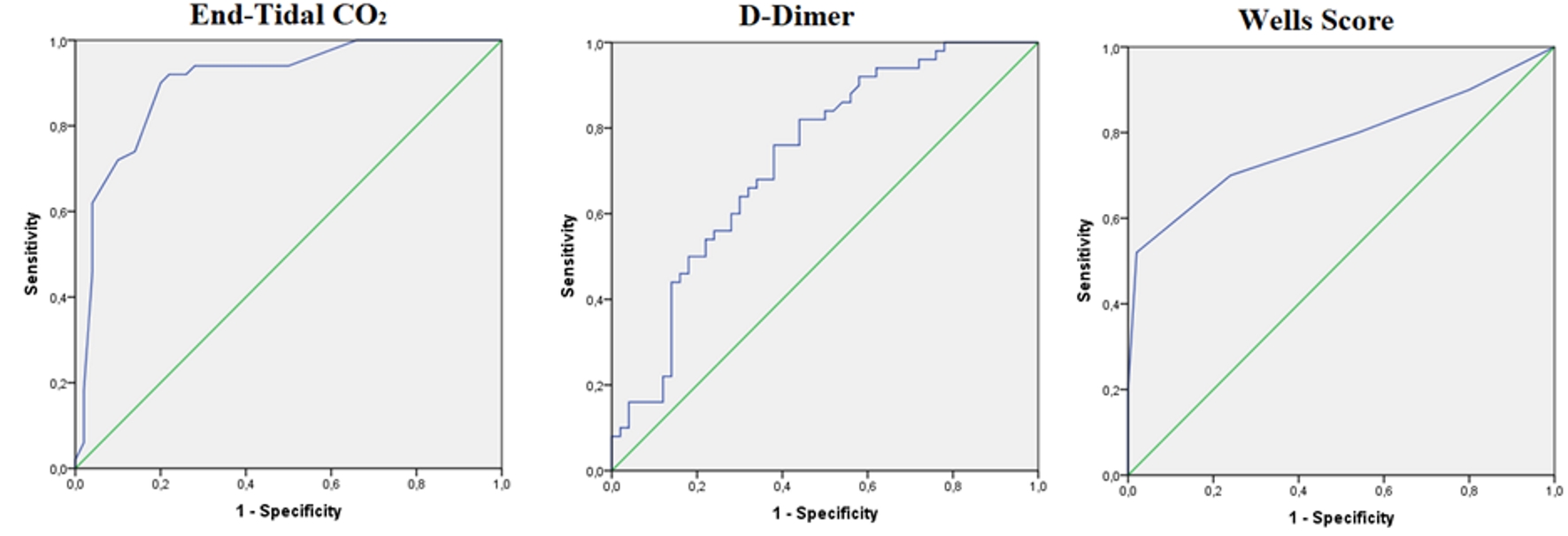 Click for large image | Figure 2. ROC analyses for ETCO2, Wells score, and D-dimer level for diagnosing PE. PE: pulmonary embolism; ETCO2: end-tidal carbon dioxide; ROC: receiver operating characteristic. |
 Click to view | Table 4. Comparison of the Sensitivities, Specificities, PPVs, and NPVs of ETCO2, Wells Score, and D-Dimer Level for Diagnosing PE |
Factors associated with PE were evaluated with logistic regression analysis. Age and sex were adjusted for the assessment of Wells score and ETCO2. The logistic regression analysis was revealed that Wells score and ETCO2 were significant predictors of PE (Table 5).
 Click to view | Table 5. Logistic Regression Analysis for PE |
| Discussion | ▴Top |
Among patients admitted to emergency departments, PE is one of the preventable causes of death. In Europe, about 300,000 deaths occur annually due to PE [8]. PE is a highly morbid and mortal disease. Unless timely and adequately treated, it may cause sudden death, and thus swift diagnosis and adequate therapy are mandatory [9]. As it exhibits variable clinical features, diagnosis of PE may be difficult, particularly for emergency physicians. Considering that PE can be confirmed in only 10-15% of patients undergoing diagnostic imaging, and the cost, workload, and possible complications of such studies, novel less invasive tools are needed to be used for diagnosing PE in ED [10, 11].
Literature data suggest that the incidence of PE increases proportionately to age, and it occurs more commonly in patients older than 60 years and among women [12]. Previous studies have shown that women suffering PE had higher Wells scores and ECG scores, greater rates of immobilization and surgical interventions, and a higher mean pulmonary artery pressures on echocardiography [13]. Our study also revealed that PE more commonly occurred in women, and the patients had a mean age of 66.3 years.
Comorbidities like respiratory failure, cerebrovascular accident (CVA), acute infectious conditions, acute coronary syndromes (ACSs), chronic heart failure (CHF), and cancer trigger PE and boost mortality [13]. In a study by Riaz et al, the most common comorbid conditions were obesity, hypertension, cancer, chronic lung disease, and cardiovascular diseases [14]. Our study also demonstrated that patients diagnosed with PE had comorbidities, in descending order of hypertension (HT), diabetes mellitus (DM), deep vein thrombosis (DVT), coronary artery disease (CAD), and CHF.
Although the definitive diagnostic method for PE is pulmonary CT angiography (CTA), scoring systems taking into account clinical probabilities serve to determine low-risk patients and avoid unnecessary diagnostic tests, thus preventing adverse clinical outcomes and heavy economic burden when used along with D-dimer as part of a proper diagnostic algorithm [15]. Wells, Geneva, and Gestalt are available scoring systems used for this purpose [1]. Wells scoring uses a three-stage risk scoring. Studies with this clinically validated scoring system have shown that as the score points increased, the probability of PE also increased; although it is incapable of making the diagnosis of PE, when combined with D-dimer level, it is highly important for excluding PE [16]. Our study demonstrated that patients deemed high-risk had a significantly higher Wells score than those without and all of them had a confirmed PE.
Many biochemical markers are used for risk stratifying and excluding PE. Among them, D-dimer, a fibrin degradation product, is decisive in clinical decision making; however, it has a low specificity due to its universal increase in thromboembolic events and unclear cut-off level [8, 17]. Nevertheless, its sensitivity approaches to 100% when combined with Wells score [18]. Perrier et al reported that D-dimer was useful for patients admitted to ED with suspected PE [19]. Wells et al reported a diagnostic confirmation rate of 46% when a Wells score of 4 or less was combined with a negative D-dimer test [20]. Our study demonstrated a significantly higher D-dimer level in the PE group than in the non-PE group. The 100% sensitivity allows us to detect virtually every person who has PE but it has relatively low specificity, meaning that it can be falsely positive for a number of patients who actually do not have PE. In this situation, the high NPV (100%) of D-dimer level can safely exclude the possibility of PE in patients with a low probability of PE. However, again a negative value is not safe for excluding PE in patients with a high clinical probability; hence, D-dimer cannot be used for making the diagnosis of PE, and the gold standard is CTPA [21].
Patients with PE show arterial blood gas changes depending on emboli size, obstruction severity, and underlying condition [22]. Hypoxemia and alveolar-arterial oxygen gradient, a more sensitive parameter than hypoxia/hypocapnia, are the most commonly observed arterial blood gas changes; AaDO2 is a more valuable parameter for diagnosing PE [23]. Arterial blood gas analysis of our patients revealed a significantly lower PO2 level in PE positive ones, as reported in the previous literature [2].
ETCO2, measured in exhaled breath and indicating mean alveolar CO2 level produced by varying levels of ventilation and perfusion in the lungs, is mostly used to verify the correct location of intubation tube in the ED [14]. However, as ETCO2 reflects dead space that defines well ventilated but poorly perfused alveoli and measurements with pulmonary capnography provide valuable information about pulmonary blood flow during alveolar ventilation, this parameter has been shown to be feasible in both diagnosis and determining treatment success in conditions affecting pulmonary blood flow like PE [5, 24]. Studies aimed to explore novel rapid and non-invasive methods have revealed that the PE diagnosis was confirmed in a high percentage of patients with a high PE clinical probability and a low ETCO2 level [2]. Sanchez et al reported that capnographically measured dead space had a sensitivity of 68.5% and a specificity of 81.5% [25]. Our study revealed a significantly lower ETCO2 level in the PE group. Furthermore, it was 28.5 mm Hg on average in the group with excluded PE. For an ETCO2 cut-off value of 34, the sensitivity was 100%, specificity 8%, PPV 52.1%, and NPV 100%. Hemnes et al reported a sensitivity of 87%, specificity of 53%, and NPV of 97% for a cut-off level of 36 mm Hg [6]. As we mentioned above, the high sensitivity, low specificity can be falsely positive for a number of patients who actually do not have PE. In this situation, the high NPV (100%) of ETCO2 level can safely exclude the possibility of PE in patients with a low probability of PE. However, capnographic measurements alone are insufficient to exclude the diagnosis of PE but serve to make a diagnosis eliminating advanced workup when combined with clinical probability [25].
Conclusions
We reached a conclusion that ETCO2 measurement, as a noninvasive technique, may be used as a diagnostic method for diagnosing PTE among patients who present to ED with suspected PE. However, we are of the opinion that larger studies are needed in this subject, and that ETCO2 measurement would have an important role in ED if those studies would support our findings.
Acknowledgments
We are deeply indebted to all study subjects for their understanding and cooperation with the study.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All patients provided written informed consent prior to study participation.
Author Contributions
MO contributed to the conception and design of the study, data analysis and writing of the manuscript; BMS contributed to the data collection, data analysis and writing of the manuscript; FY contributed to the design of the study, data interpretation and editing of the manuscript; AY contributed to the design of the study, data interpretation and editing of the manuscript; MD and SK contributed to the design of the study, data interpretation and editing of the manuscript.
| References | ▴Top |
- ED Arslan, SA Yesilaras, C Kavalci, S Bozkurt, F Yılmaz, T Durdu, et al. Prediction Of Pretest Probability Scoring Systems In Pulmonary Embolism: Wells, Kline And Geneva. Int J Clin Med. 2012;3:731-735.
doi - Rumpf TH, Krizmaric M, Grmec S. Capnometry in suspected pulmonary embolism with positive D-dimer in the field. Crit Care. 2009;13(6):R196.
doi pubmed - Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(45):3145-3146.
doi - Kline JA, Meek S, Boudrow D, Warner D, Colucciello S. Use of the alveolar dead space fraction (Vd/Vt) and plasma D-dimers to exclude acute pulmonary embolism in ambulatory patients. Acad Emerg Med. 1997;4(9):856-863.
doi pubmed - Verschuren F, Liistro G, Coffeng R, Thys F, Roeseler J, Zech F, Reynaert M. Volumetric capnography as a screening test for pulmonary embolism in the emergency department. Chest. 2004;125(3):841-850.
doi pubmed - Hemnes AR, Newman AL, Rosenbaum B, Barrett TW, Zhou C, Rice TW, Newman JH. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Respir J. 2010;35(4):735-741.
doi pubmed - Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135(2):98-107.
doi pubmed - Keller K, Beule J, Schulz A, Coldewey M, Dippold W, Balzer JO. D-dimer for risk stratification in haemodynamically stable patients with acute pulmonary embolism. Adv Med Sci. 2015;60(2):204-210.
doi pubmed - Beydilli I, Yilmaz F, Sonmez BM, Kozaci N, Yilmaz A, Toksul IH, Guven R, et al. Thrombolytic therapy delay is independent predictor of mortality in acute pulmonary embolism at emergency service. Kaohsiung J Med Sci. 2016;32(11):572-578.
doi pubmed - Hendriksen JM, Geersing GJ, Lucassen WA, Erkens PM, Stoffers HE, van Weert HC, Buller HR, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438.
doi pubmed - Bach AG, Bandzauner R, Nansalmaa B, Schurig N, Meyer HJ, Taute BM, Wienke A, et al. Timing of pulmonary embolism diagnosis in the emergency department. Thromb Res. 2016;137:53-57.
doi pubmed - Duru S, Ergun R, Dilli A, Kaplan T, Kaplan B, Ardic S. [Clinical, laboratory and computed tomography pulmonary angiography results in pulmonary embolism: retrospective evaluation of 205 patients]. Anadolu Kardiyol Derg. 2012;12(2):142-149.
- Dursunoglu N, Baser S, Dursunoglu D, Moray A, Kiter G, Ozkurt S, Evyapan F, et al. [Differences between men and women in the clinical and laboratory findings of patients diagnosed with pulmonary embolism]. Tuberk Toraks. 2007;55(3):246-252.
- Riaz I, Jacob B. Pulmonary embolism in Bradford, UK: role of end-tidal CO2 as a screening tool. Clin Med (Lond). 2014;14(2):128-133.
doi pubmed - Sanjuan P, Rodriguez-Nunez N, Rabade C, Lama A, Ferreiro L, Gonzalez-Barcala FJ, Alvarez-Dobano JM, et al. Probability scores and diagnostic algorithms in pulmonary embolism: are they followed in clinical practice? Arch Bronconeumol. 2014;50(5):172-178.
doi pubmed - Shen JH, Chen HL, Chen JR, Xing JL, Gu P, Zhu BF. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41(3):482-492.
doi pubmed - Kara H, Bayir A, Degirmenci S, Kayis SA, Akinci M, Ak A, Celik B, et al. D-dimer and D-dimer/fibrinogen ratio in predicting pulmonary embolism in patients evaluated in a hospital emergency department. Acta Clin Belg. 2014;69(4):240-245.
doi pubmed - van Es J, Beenen LF, Douma RA, den Exter PL, Mos IC, Kaasjager HA, Huisman MV, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13(8):1428-1435.
doi pubmed - Perrier A, Desmarais S, Miron MJ, de Moerloose P, Lepage R, Slosman D, Didier D, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353(9148):190-195.
doi - Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AG, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
doi pubmed - Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, Bengel F, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276-2315.
doi pubmed - Ince O, Altintas N, Findik S, Sariaydin M. Risk stratification in submassive pulmonary embolism via alveolar-arterial oxygen gradient. Hippokratia. 2014;18(4):333-339.
- McFarlane MJ, Imperiale TF. Use of the alveolar-arterial oxygen gradient in the diagnosis of pulmonary embolism. Am J Med. 1994;96(1):57-62.
doi - Chopin C, Fesard P, Mangalaboyi J, Lestavel P, Chambrin MC, Fourrier F, Rime A. Use of capnography in diagnosis of pulmonary embolism during acute respiratory failure of chronic obstructive pulmonary disease. Crit Care Med. 1990;18(4):353-357.
doi pubmed - Sanchez O, Wermert D, Faisy C, Revel MP, Diehl JL, Sors H, Meyer G. Clinical probability and alveolar dead space measurement for suspected pulmonary embolism in patients with an abnormal D-dimer test result. J Thromb Haemost. 2006;4(7):1517-1522.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


