| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 1, January 2018, pages 22-26
Safety of Landiolol Hydrochloride as a Premedication for Producing an Appropriate Heart Rate for Multidetector-Row Computed Tomography Coronary Angiography
Rie Koyoshia, e, Yuhei Shigab, e, Yoshiaki Idemotob, Yoko Uedab, Kohei Tashirob, Takashi Kuwanob, Ken Kitajimab, Kanta Fujimib, c, Akira Kawamurab, Masahiro Ogawab, Shin-ichiro Miurab, d, f
aDivision of Medical Safety Management, Fukuoka University Hospital, Fukuoka, Japan
bDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan
cDivision of Rehabilitation, Fukuoka University Hospital, Fukuoka, Japan
dDepartment of Molecular Cardiovascular Therapeutics, Fukuoka University School of Medicine, Fukuoka, Japan
eThese authors contributed equally to this work.
fCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-Ku, Fukuoka 814-0180, Japan
Manuscript submitted September 30, 2017, accepted October 12, 2017
Short title: Landiolol Hydrochloride and Coronary Angiography
doi: https://doi.org/10.14740/jocmr3213w
| Abstract | ▴Top |
Background: We evaluated the safety of a bolus injection of landiolol hydrochloride, an ultrashort-acting β1-selective antagonist, as a premedication prior to multidetector-row computed tomography coronary angiography (CTA).
Methods: The subjects consisted of 176 patients (M/F = 64:112, 67 ± 11 years) who had heart rate (HR) at rest ≥ 70 beats/min (bpm) and underwent CTA. Systolic/diastolic blood pressure (SBP/DBP) and HR were measured before and after the administration of landiolol.
Results: SBP/DBP and HR upon entry to the CT room were 136 ± 17/80 ± 11 mm Hg and 83 ± 10 bpm, respectively. HR was significantly reduced at the time of CTA scan (62 ± 7 bpm). Next, we divided the patients into three groups according to HR upon entry to the CT room: 70 - 79 bpm (n = 76), 80 - 89 bpm (n = 60) and ≥ 90 bpm (n = 40). HR at the time of CTA scan was significantly lower than that upon entry to the CT room in all three groups: 70 - 79 bpm (74 ± 3 bpm upon entry to the CT room to 61 ± 6 bpm at the time of CAT scan), 80 - 89 bpm (84 ± 3 to 63 ± 7 bpm) and ≥ 90 bpm (98 ± 6 to 65 ± 7 bpm). Although SBP/DBP was significantly decreased after the CTA scan (123 ± 18/72 ± 12 mm Hg), landiolol had no severe adverse events throughout CTA.
Conclusion: In conclusion, a bolus injection of landiolol reduced HR by about 20 bpm without any severe adverse effects. Thus, a bolus injection of landiolol hydrochloride may be a suitable pretreatment for controlling HR in CTA.
Keywords: Blood pressure; Heart rate; Hypotension; Adverse events
| Introduction | ▴Top |
Multidetector-row computed tomography (MDCT) coronary angiography (CTA) has become more widely available in many general hospitals, and enables the accurate non-invasive assessment of coronary artery stenosis [1], calcification [2], and plaque [3]. A higher heart rate (HR) during CTA can evoke motion artifacts that reduce the imaging quality [4]. Oral β-blockers have been widely used as premedication to reduce the HR to a level suitable for CTA [5, 6].
Recently, intravenous β-blockers have been used to reduce the HR for CTA [7-9]. Landiolol hydrochloride, an ultrashort-acting β1-selective antagonist, exerts a clinically relevant negative chronotropic effect and can be used to safely reduce HR [7-9]. These previous studies used 16- and 64-row CT scanners. In this study, we evaluated the safety of landiolol hydrochloride during CTA using a 320-row CT scanner.
| Materials and Methods | ▴Top |
Study population
We enrolled 176 subjects who had HR at rest ≥ 70 beats/min (bpm) and underwent CTA. CTA was performed in subjects who were clinically suspected of having coronary artery disease (CAD) or who had at least one cardiac risk factor. Patients with Cr > 2.0 mg/dL or contrast-induced allergy did not undergo CTA. The protocol in this study was approved by the ethics committee of Fukuoka University Hospital (IRB #11-06(09-089)), and all subjects gave their informed consent to participate.
Evaluation of coronary stenosis using CTA
We evaluated coronary stenosis using CTA as previously described [1]. Subjects were scanned by 320-MDCT on an Aquilion ONE ViSION (TOSHIBA, Tokyo, Japan). The use of landiolol hydrochloride [4R-2,2-dimethyl-1,3-dioxolan-4-yl]methyl 3-[4-[(2S)-2-hydroxy-3-[2-(morpholine-4-carbonylamino) ethylamino]propoxy]phenyl] propanoate hydrochloride (Ono Pharmaceutical Co. Ltd, Japan), before scanning was approved by a physician. After the use of landiolol was approved, patients received a bolus injection of landiolol as a pretreatment when the HR at rest ≥ 70 bpm. Landiolol was injected intravenously 4 min before starting CTA. In CTA, 21.5 mgI/kg/s contrast medium (Iopamiron, 370 mg iodine/mL; Bayer Yakuhin. Ltd, Osaka, Japan) equivalent to the patient’s body weight × 0.7 mL was injected over 10 s, followed by 35 mL contrast agent and 30 mL saline solution, each at a flow rate of 1.8 mL/s, with a dual injector.
The region of interest was placed within the ascending aorta, and the scan was started when the CT density reached 100 Hounsfield Units higher than the baseline CT density. The scan was performed between the tracheal bifurcation and diaphragm with the following parameters: 320-MDCT-collimation width 0.5 mm, rotation speed 0.275 s/rotation, tube voltage 120 kV, and auto tube current.
Overall, 15 coronary artery segments were assessed in all patients. Narrowing of the normal contrast-enhanced lumen to ≥ 50% that could be identified in multiplanar reconstructions or cross-sectional images was defined as significant stenosis in CAD.
Evaluation of risk factors for CAD
Data regarding body mass index (BMI), systolic blood pressure (SBP), diastolic BP (DBP), serum levels of triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), fasting glucose, hemoglobin A1c, estimated glomerular filtration rate (eGFR), smoking status (current versus non-smoker), family history (myocardial infarction, angina pectoris or sudden death) and medication use were collected.
BMI was calculated as weight (kg)/height (m)2. BP was determined as the mean of two measurements obtained in an office setting by the conventional cuff method using a mercury sphygmomanometer after at least 5 min of rest. All of the blood samples were drawn in the morning after the patients had fasted overnight. The characteristics of the patients with regard to history of hypertension (HTN), dyslipidemia (DL) and diabetes mellitus (DM) were obtained from medical records. Patients who had a current SBP/DBP ≥ 140/90 mm Hg or who were receiving antihypertensive therapy were considered to have HTN. Patients with LDL-C ≥ 140 mg/dL, TG ≥ 150 mg/dL, and/or HDL-C < 40 mg/dL, or who were receiving lipid-lowering therapy were considered to have DL [10]. DM was defined using the American Diabetes Association criteria [11] or the administration of a glucose-lowering drug.
Statistical analysis
A statistical analysis was performed using the Stat View statistical software package (Stat View 5; SAS Institute Inc., Cary, NC, USA). Continuous variables are shown as the mean ± standard deviation. Categorical and continuous variables were compared between the groups by a Chi-square analysis and t-test, respectively. A value of P < 0.05 was considered significant.
| Results | ▴Top |
Patient characteristics
Table 1 shows the characteristics of the 176 patients, who consisted of 64 (36%) men and 112 (64%) women. The frequencies of HTN, DM, DL, CKD and CAD in all patients were 66%, 21%, 61%, 11% and 39%, respectively. The mean age was 67 ± 11 years and BMI was 24.1 ± 3.7 kg/m2. Patients received a bolus injection of landiolol hydrochloride (16.1 ± 7.4 mg) as a pretreatment. The percentages of the use of angiotensin II receptor blocker (ARB)/angiotensin-converting enzyme inhibitor (ACEI), β-blocker, calcium channel blocker (CCB) and statin were 35%, 2%, 41% and 35%, respectively (Table 2).
 Click to view | Table 1. Patient Characteristics |
 Click to view | Table 2. Medications (n = 176) |
SBP, DBP, HR and adverse effects before and after landiolol injection
SBP, DBP and HR were measured before and after the administration of landiolol. Figure 1a shows the time-course changes in HR in all patients. HRs upon entry to the CT room, before landiolol injection, after injection at the time of the CTA scan and after the CTA scan were 83 ± 10, 83 ± 10, 62 ± 7, and 69 ± 8 bpm, respectively. HR was significantly reduced at the time of the CTA scan. Next, we divided the patients into three groups according to HR upon entry to the CT room: 70 - 79 bpm (n = 76), 80 - 89 bpm (n = 60) and ≥ 90 bpm (n = 40) (Fig. 1b). The total doses of landiolol injected in the 70 - 79 bpm, 80 - 89 bpm and ≥ 90 bpm groups were 12.2 ± 5.3, 17.9 ± 6.9 and 20.9 ± 7.6 mg, respectively. HR at the time of the CTA scan was significantly lower than that upon entry to the CT room in all three groups: 70 - 79 bpm (HR 74 ± 3 bpm upon entry to the CT room to 61 ± 6 bpm at the time of the CAT scan), 80 - 89 bpm (84 ± 3 to 63 ± 7 bpm) and ≥ 90 bpm (98 ± 6 to 65 ± 7 bpm).
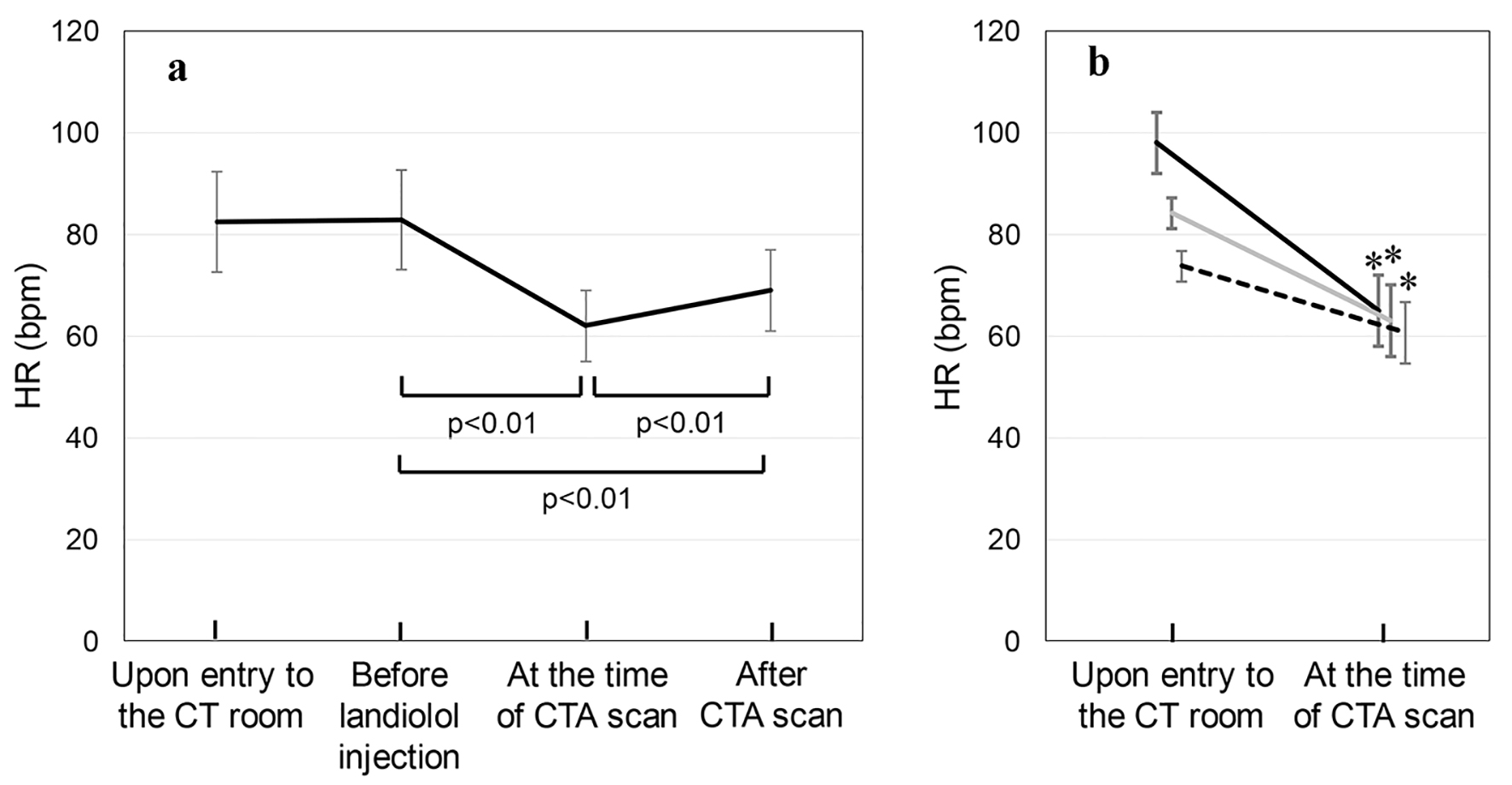 Click for large image | Figure 1. (a) HR upon entry to the CT room, before the injection of landiolol hydrochloride, after injection at the time of the CTA scan and after the CTA scan in all patients. (b) The patients were divided into three groups according to HR upon entry to the CT room: 70 - 79 bpm (n = 76, dotted line), 80 - 89 bpm (n = 60, gray line) and ≥ 90 bpm (n = 40, solid line) groups. *P < 0.01 vs. upon entry to the CT room. |
Overall, SBP/DBP upon entry to the CT room and after the CTA scan was 136 ± 17/80 ± 12 and 123 ± 18/72 ± 12 mm Hg, respectively. SBP/DBP upon entry to the CT room was significantly lower than that after the CTA scan. Among 176 patients, eight patients (4.5%) had adverse events (two patients had floating felling, two nausea, one vomiting, one bad feeling, one hypotension, and one lightheadedness) throughout CTA. There were no severe adverse events that required administration of the study drug to be terminated.
| Discussion | ▴Top |
Landiolol hydrochloride, a β1-selective agent, was used in this study to reduce HR during CTA. We found that a bolus injection of landiolol hydrochloride sufficiently reduced HR for this purpose. Since there were no severe adverse effects, a bolus injection of landiolol hydrochloride appears to be a suitable pretreatment for controlling HR during CTA.
Landiolol hydrochloride is metabolized very quickly in the liver to an inactive metabolite with a short t1/2 of 4 min. It has a very rapid onset and offset of action and is highly selective for β1-receptors [12]. These features result in fewer side-effects, such as bronchial asthma or peripheral vasoconstriction, than with other longer-acting β1-adrenergic antagonists. It has been shown to be both safe and effective for urgent use in patients with tachyarrhythmia during the perioperative phase [13]. In addition, intravenous administration of landiolol hydrochloride has been used as a premedication for producing an appropriate HR for CTA without any adverse hemodynamic or physical effects [8, 9, 14]. Although premedication of landiolol hydrochloride resulted in significant reduction of BP, there were no serious adverse effects throughout CTA in all patients.
Adequate reduction of HR is the most important manipulation for avoiding motion artifacts and improving image quality, and the well-controlled reduction of HR has been reported not only to provide better image quality but also to minimize radiation exposure [15]. In this study, HR upon entry to the CT room (83 ± 10 bpm) was significantly reduced at the time of the CTA scan (62 ± 7 bpm). The HR ≥ 90 bpm group (HR 98 ± 6 bpm upon entry to the CT room) showed a significant reduction of HR at the time of the CTA scan (HR 65 ± 7 bpm), although this group required higher doses of landiolol hydrochloride than the 70 - 79 bpm and 80 - 89 bpm groups. Overall, landiolol hydrochloride induced a 20 bpm reduction in HR, and this was enough to avoid motion artifacts and to improve image quality in CTA.
Study limitations
This study has several limitations. First, the study enrolled a relatively small number of patients. Second, CTA was performed after various treatments. We did not confirm the accuracy of the diagnosis, although this has been reported previously [9]. Large-scale studies will be needed to clarify these limitations.
Conclusion
The present results suggest that bolus injection of landiolol hydrochloride is a suitable pretreatment for controlling HR during CTA.
Funding
None.
Conflict of Interest
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. SM belongs to the Department of Molecular Cardiovascular Therapeutics, which is supported by MSD, Co. LTD.
| References | ▴Top |
- Mitsutake R, Niimura H, Miura S, Zhang B, Iwata A, Nishikawa H, Kawamura A, et al. Clinical significance of the coronary calcification score by multidetector row computed tomography for the evaluation of coronary stenosis in Japanese patients. Circ J. 2006;70(9):1122-1127.
doi pubmed - Nitta K, Akiba T, Suzuki K, Uchida K, Ogawa T, Majima K, Watanabe R, et al. Assessment of coronary artery calcification in hemodialysis patients using multi-detector spiral CT scan. Hypertens Res. 2004;27(8):527-533.
doi pubmed - Achenbach S, Ropers D, Hoffmann U, MacNeill B, Baum U, Pohle K, Brady TJ, et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43(5):842-847.
doi pubmed - Achenbach S, Giesler T, Ropers D, Ulzheimer S, Derlien H, Schulte C, Wenkel E, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography. Circulation. 2001;103(21):2535-2538.
doi pubmed - Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761-1791.
doi pubmed - Shim SS, Kim Y, Lim SM. Improvement of image quality with beta-blocker premedication on ECG-gated 16-MDCT coronary angiography. AJR Am J Roentgenol. 2005;184(2):649-654.
doi pubmed - Hirano M, Yamashina A, Hara K, Ikari Y, Jinzaki M, Iino M, Yamaguchi T, et al. A randomized, double-blind, placebo-controlled, phase III study of the short-acting beta1-adrenergic receptor blocker landiolol hydrochloride for coronary computed tomography angiography in Japanese patients with suspected ischemic cardiac disease. Clin Drug Investig. 2014;34(1):53-62.
doi pubmed - Hirano M, Yamashina A, Hara K, Ikari Y, Jinzaki M, Iino M, Yamaguchi T, et al. A multicenter, open-label study of an intravenous short-acting beta1-adrenergic receptor antagonist landiolol hydrochloride for coronary computed tomography angiography by 16-slice multi-detector computed tomography in Japanese patients with suspected ischemic cardiac disease. Drugs R D. 2014;14(3):185-194.
doi pubmed - Osawa K, Miyoshi T, Sato S, Akagi N, Morimitsu Y, Nakamura K, Kohno K, et al. Safety and efficacy of a bolus injection of landiolol hydrochloride as a premedication for multidetector-row computed tomography coronary angiography. Circ J. 2013;77(1):146-152.
doi pubmed - The Japan Atherosclerosis Society. Chapter 3. Goals of dyslipidemia management. J Atheroscler Thromb. 2009;16 Sup:15-25.
- American Diabetes A. Screening for type 2 diabetes. Diabetes Care. 2004;27(Suppl 1):S11-14.
- Atarashi H, Kuruma A, Yashima M, Saitoh H, Ino T, Endoh Y, Hayakawa H. Pharmacokinetics of landiolol hydrochloride, a new ultra-short-acting beta-blocker, in patients with cardiac arrhythmias. Clin Pharmacol Ther. 2000;68(2):143-150.
doi pubmed - Mizuno J, Yoshiya I, Yokoyama T, Yamada Y, Arita H, Hanaoka K. Age and sex-related differences in dose-dependent hemodynamic response to landiolol hydrochloride during general anesthesia. Eur J Clin Pharmacol. 2007;63(3):243-252.
doi pubmed - Isobe S, Sato K, Sugiura K, Mimura T, Kobayashi M, Meno C, Kato M, et al. Feasibility of intravenous administration of landiolol hydrochloride for multislice computed tomography coronary angiography: initial experience. Circ J. 2008;72(11):1814-1820.
doi pubmed - Jakobs TF, Becker CR, Ohnesorge B, Flohr T, Suess C, Schoepf UJ, Reiser MF. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002;12(5):1081-1086.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


