| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 9, Number 6, June 2017, pages 488-498
Phosphodiesterase-5 Inhibitors Improve Clinical Outcomes, Exercise Capacity and Pulmonary Hemodynamics in Patients With Heart Failure With Reduced Left Ventricular Ejection Fraction: A Meta-Analysis
Renato De Vecchisa, c, Arturo Cesarob, Carmelina Arianoa, Anna Giasia, Carmela Cioppaa
aCardiology Unit, Presidio Sanitario Intermedio “Elena d’Aosta”, ASL Napoli 1 Centro, via Cagnazzi 29, 80137 Napoli, Italy
bDepartment of Cardiology, Second University of Napoli, Monaldi Hospital, via Leonardo Bianchi 1, 80131 Napoli, Italy
cCorresponding Author: Renato De Vecchis, via P.Gaurico 21, 80125 Napoli, Italy
Manuscript accepted for publication March 27, 2017
Short title: PDE5 Inhibitors for the Treatment of CHF
doi: https://doi.org/10.14740/jocmr3008w
| Abstract | ▴Top |
Background: Several studies have compared the use of phosphodiesterase-5 (PDE5) inhibitors sildenafil or udenafil with the placebo in patients suffering from pulmonary hypertension (PH) due to left chronic heart failure (CHF), corresponding to group 2 (PH due to left heart disease) of the PH classification (according to 2015 ESC/ERS guidelines for the diagnosis and treatment of PH). The results of the use of PDE5 inhibitors in the PH due to left heart disease were inconsistent and heterogeneous. Therefore, we carried out a meta-analysis to assess the effect of PDE5 inhibitors in this clinical setting, i.e., patients with left CHF.
Methods: A systematic search was conducted using the PubMed and Embase electronic archives. Studies had to be prospective randomized controlled trials (RCTs). In each of the RCTs admitted to meta-analysis, a comparison was made between a group of CHF patients taking a PDE5 inhibitor and a second group assigned a placebo. Studies were incorporated in the meta-analysis provided that they had sufficient information about two or more of the following clinical, ergospirometric or hemodynamic outcomes: the composite of all-cause death and hospitalization, adverse events, peak VO2, 6-min walking distance (6MWD), left ventricular ejection fraction (LVEF), E/e’ ratio, mean pulmonary arterial pressure (mPAP), pulmonary arterial systolic pressure (PASP), and pulmonary vascular resistance (PVR).
Results: Fourteen studies enrolling a total of 928 patients were incorporated in the meta-analysis. Among them,13 were RCTs and one was a subgroup analysis. Among patients with CHF with reduced left ventricular ejection fraction (HFREF, n = 555), a significant benefit was conferred by PDE5 inhibitors against the risk of the composite endpoint of death and hospitalizations (odds ratio (OR): 0.28; 95% confidence interval (CI): 0.10 - 0.74; P = 0.03). Furthermore, among HFREF patients, PDE5 inhibitors were associated with a significant improvement in peak VO2 (difference in means (MD): 3.76 mL/min/kg; 95% CI: 3.27 - 4.25) as well as in 6MWD (MD: 22.7 m; 95% CI: 8.19 - 37.21) and LVEF (MD: 4.30%; 95% CI: 2.18% to 6.42%). For patients with HFREF, PDE5 inhibitors caused a non-significant reduction in mPAP, while PASP was significantly reduced (MD: -11.52 mm Hg; 95% CI: -15.56 to -7.49; P < 0.001). By contrast, in the RCTs of patients with CHF with preserved left ventricular ejection fraction (HFpEF, n = 373), no benefit ensued from PDE5 inhibitor use regarding all of the investigated clinical, ergospirometric or hemodynamic endpoints.
Conclusions: PDE5 inhibitors improved clinical outcomes, exercise capacity and pulmonary hemodynamics in patients with HFREF, but not in HFpEF. However, considering the relatively small size of the HFpEF subset enrolled so far in the RCTs that explored the PDE5 inhibitor effects, further research in this field is undoubtedly warranted.
Keywords: Sildenafil; Phosphodiesterase-5 inhibitors; Heart failure; Clinical outcomes; Ergospirometry; Pulmonary hemodynamics; Meta-analysis
| Introduction | ▴Top |
The cardinal symptom of heart failure, i.e., the dyspnea, is largely attributable to pulmonary hypertension (PH) and congestion in the pulmonary vasculature [1]. So it is crucial to emphasize the very important role that PH plays in causing the symptoms and the clinical picture of heart failure either right-sided or left-sided or biventricular. PH associated with left heart disease (PH-LHD) coincides with the group 2 of the most recent International Classification of the Pulmonary Hypertension [2]. The favorable effects of phosphodiesterase-5 (PDE5) inhibitors, in particular sildenafil, in the treatment of PH are mainly attributed to the action exerted on the pulmonary arteriolar - precapillary district (so-called “precapillary pulmonary selectivity” of PDE5 inhibitors) [3, 4]. In other words, the benefit of PDE5 inhibitors in treating heart failure may originate from their hemodynamic effect for the combined post- and pre-capillary PH (Cpc-PH), but not for the isolated post-capillary PH (Ipc-PH) [5].
Aims
In the present article, in order to evaluate the effects exercised by sildenafil or other PDE5 inhibitors on some functional, hemodynamic or clinical endpoints, a number of meta-analyses were separately conducted in patients with chronic heart failure with reduced (HFREF) or preserved (HFpEF) left ventricular ejection fraction (LVEF), respectively.
| Methods | ▴Top |
Study selection
A systematic search using some related terms was conducted using the PubMed and Embase electronic archives. We limited our search to adults (> 18 years old) and to randomized controlled trials (RCTs). The study was performed according to the guidelines and recommendations expressed in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [6] statement. Search terms firstly included “heart failure”, “sildenafil”, “vardenafil”, “tadalafil”, “avanafil”, “udenafil”, “phosphodiesterase 5 inhibitors”, “phosphodiesterase type 5 inhibitors”, “PDE5 inhibitors”, “cardiac dysfunction”, and “pulmonary hypertension”, variously combined by means of the Boolean operators “AND” and “OR”. Roots and variants of the search terms were also used. Studies had to be prospective RCTs. In each of the studies admitted to meta-analysis, a comparison had to be made between a group of CHF patients taking a PDE5 inhibitor and a second group assigned a placebo. Studies were incorporated in the meta-analysis provided that they had sufficient information about the explored hemodynamic and/or ergospirometric and/or clinical outcomes.
Study endpoints
The included RCTs were assessed for the following outcomes: exercise capacity (peak VO2 and 6-min walking distance (6MWD)), cardiac performance (LVEF, %), diastolic function (E/e’ ratio), and pulmonary resistance (mean pulmonary arterial pressure (mPAP, mm Hg), pulmonary arterial systolic pressure (PASP, mm Hg), and pulmonary vascular resistance (PVR, dyn·sec/cm5)). Clinical outcomes were assessed as all-cause death and hospitalization and adverse events.
Data extraction
All authors participated in determining the eligibility of candidate trials. The search included publications up to June 2016 and no lower date limit was applied. Titles and abstracts of all identified citations were reviewed independently by two authors (RDV and CA). Any candidate study was selected for further screening of the full text. In the event of a possible disagreement during data extraction, the intervention of a third reviewer (AC) was scheduled to solve any conflicting interpretation. Notably, it was decided that the studies selected for the meta-analysis should have included patients aged over 18 years. In addition, animal experimental studies as well as case reports of PDE5 inhibitor administration without a control group were eliminated from the meta-analysis. Similarly, all studies not written in English, duplicated studies, review articles, editorials and expert opinions were excluded.
Quality assessment
The authors assessed the risk of bias for the recruited RCTs using the Cochrane Collaboration Risk of Bias Tool. The following risks of bias were evaluated: 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessment; 5) incomplete outcome data; and 6) other bias.
Statistical analysis
In the case of dichotomous variables, e.g., the composite of “death and hospitalizations” or adverse events, the effect size was expressed as odds ratio (OR) with a 95% confidence interval (CI), using Mantel-Haenszel method as the weighting method. When the endpoint was a continuous variable, such as “change in mPAP” or “change in 6-min walking test”, the effect size was expressed as a difference in means (MD) with a 95% CI, using inverse variance as the weighting method. Due to the large variety of patients, the effect size was calculated using a random effects model, even in case no heterogeneity was found. Statistical heterogeneity across studies was tested using Cochran’s Q test and I2 statistic (coefficient of variability due to inter-study variability). Statistical analyses were performed using RevMan 5.3 software (available from the Cochrane Collaboration; http//www.cochrane.org) and Stata version 10 (Stata Corp LP, College Station, TX, USA).
| Results | ▴Top |
In our meta-analyses, 14 studies were incorporated on the whole (Fig. 1 and Tables 1 and 2). Among them, 13 were RCTs [3, 7-16, 18, 19] and one was a subgroup analysis [17]. Patients affected by HFREF included in our meta-analysis were 555. All of them were derived from the pooling of nine RCTs plus the afore-mentioned subanalysis study (Tables 1 and 2). Conversely, patients with HFpEF included in our meta-analysis were 373 on the whole. This value corresponds to the sum of the patients enrolled by four RCTs [8, 11, 14, 18], specifically aimed to explore the effects of PDE5 inhibitors in HFpEF.
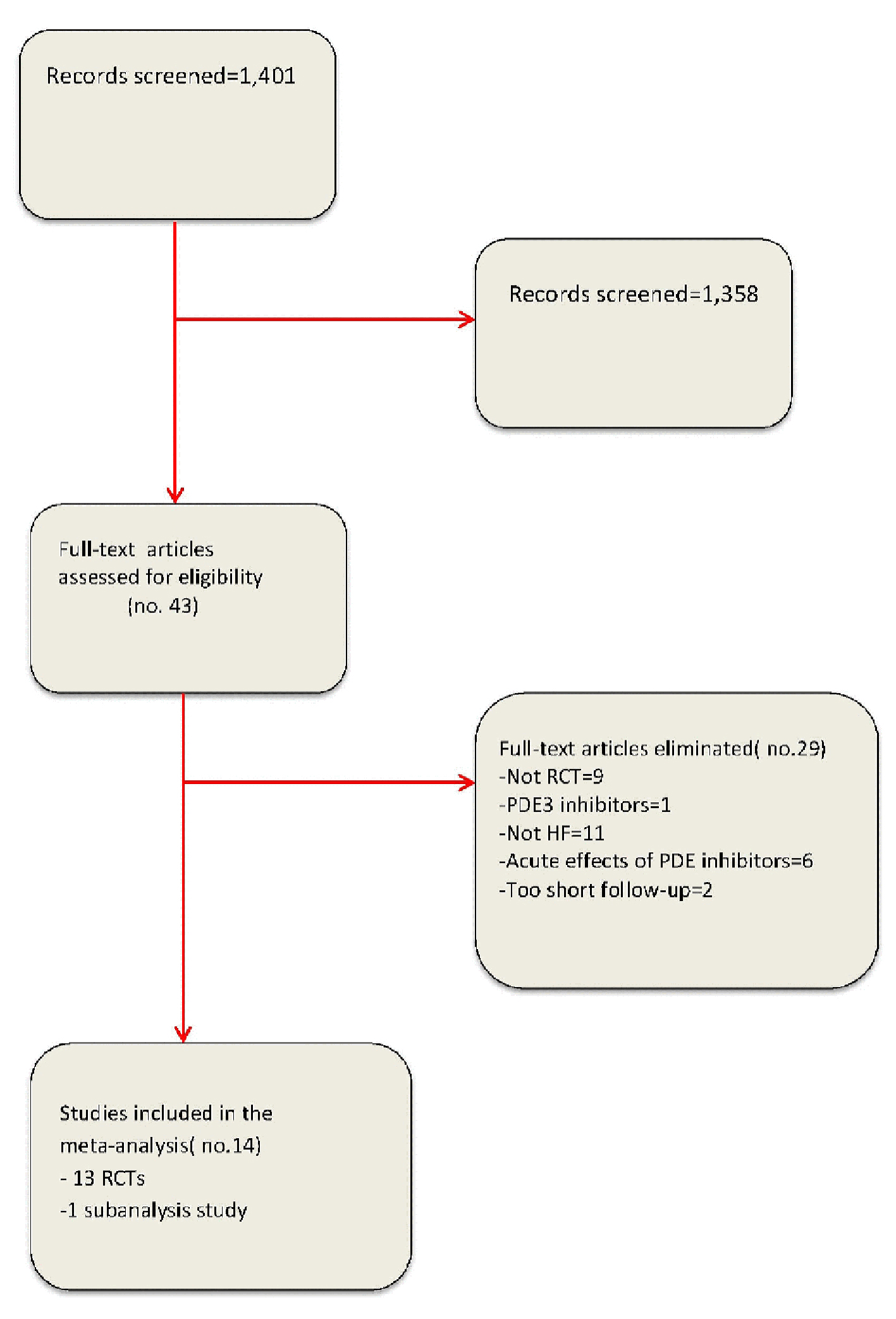 Click for large image | Figure 1. Flow diagram for meta-analysis according to PRISMA statement. |
 Click to view | Table 1. Baseline Features of Included RCTs |
 Click to view | Table 2. Different Impact of PDE5 Inhibitors According to Pulmonary Hemodynamics |
Therefore, a total of 928 patients with chronic heart failure (CHF) were considered for the elaboration of the meta-analyses conducted in the course of our research. Among the included studies, 444 patients were assigned to sildenafil (with 443 patients assigned to placebo), and 21 patients were assigned to udenafil (with 20 patients assigned to placebo) (Tables 1 and 2).
Clinical outcomes (death and/or hospitalizations and adverse events)
Seven RCTs of HFREF [3, 7, 12, 13, 15, 16, 19] reported clinical outcomes, with five hospitalization events occurring in the PDE5 inhibitor arm and 17 occurring in the control arm. These results indicate a significant benefit conferred by PDE5 inhibitors against hospitalization (OR: 0.28; 95% CI: 0.10 - 0.74; P = 0.01; Fig. 2). Among the three RCTs concerning HFpEF that had included the endpoints of death and hospitalizations, one study [11] did not report any event, whereas the remaining two studies [14, 18] signaled 16 hospitalization events on the whole occurring in the PDE5 inhibitor arm and 18 occurring in the control arm (OR: 0.81; 95% CI: 0.41 - 1.63; P = 0.56; Fig. 2). During the follow-up period, five deaths were reported.
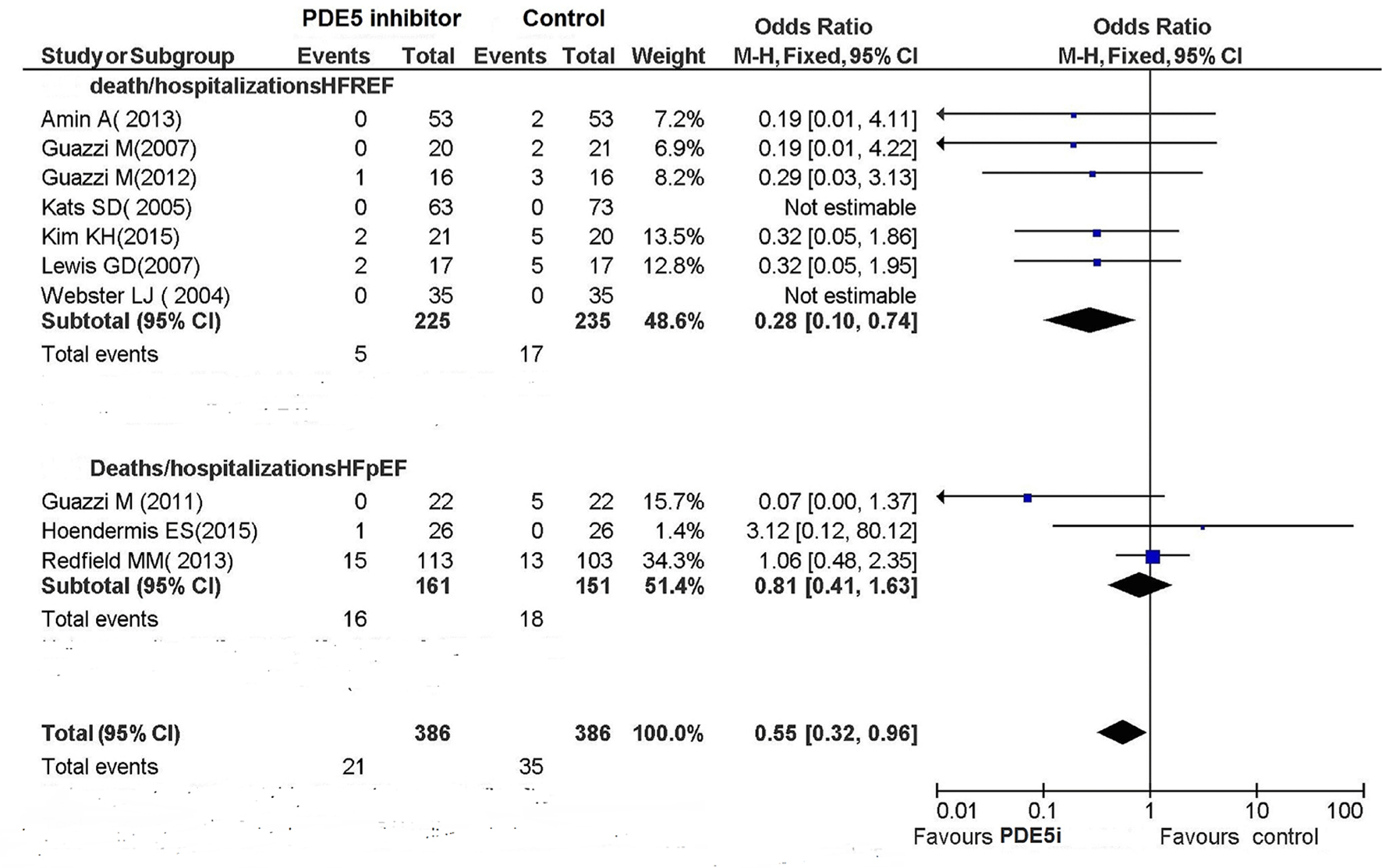 Click for large image | Figure 2. Deaths/hospitalizations of HF. |
The occurrence of adverse events in these studies did not significantly differ between the PDE5 inhibitor arm and the control arm (Fig. 3).
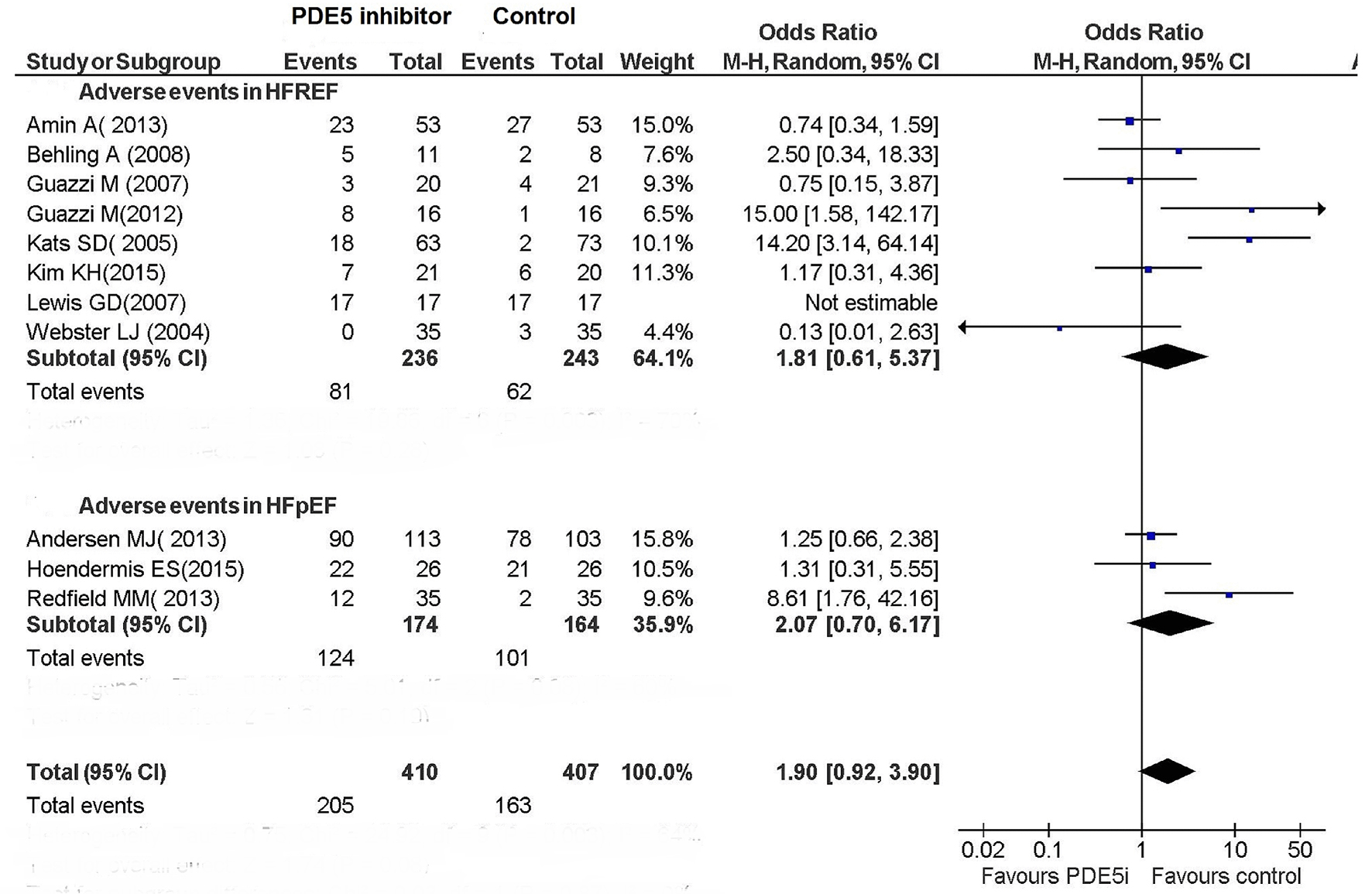 Click for large image | Figure 3. Adverse events in patients with CHF. |
Exercise capacity and cardiac performance
The use of PDE5 inhibitor significantly improved exercise capacity in patients with HFREF (Figs. 4 and 5). In particular, among the six RCTs that had investigated the peak VO2 in HFREF patients [3, 9-10, 12, 13, 16] this parameter was improved by the use of PDE5 inhibitors (difference in means (MD): 3.76; 95% CI: 3.27 - 4.25; P < 0.00001; Fig. 4). Similarly, based on the results of two studies [3, 7], in HFREF patients PDE5 inhibitor use yielded a significant betterment of 6MWD compared to placebo arm (MD: 22.7 m; 95% CI: 8.19 - 37.21; P = 0.002; Fig. 5). By contrast, in the RCTs of patients with HFpEF no benefit ensued from PDE5 inhibitor use regarding exercise capacity as measured by cardiopulmonary exercise test or 6 MWD (Figs. 4 and 5).
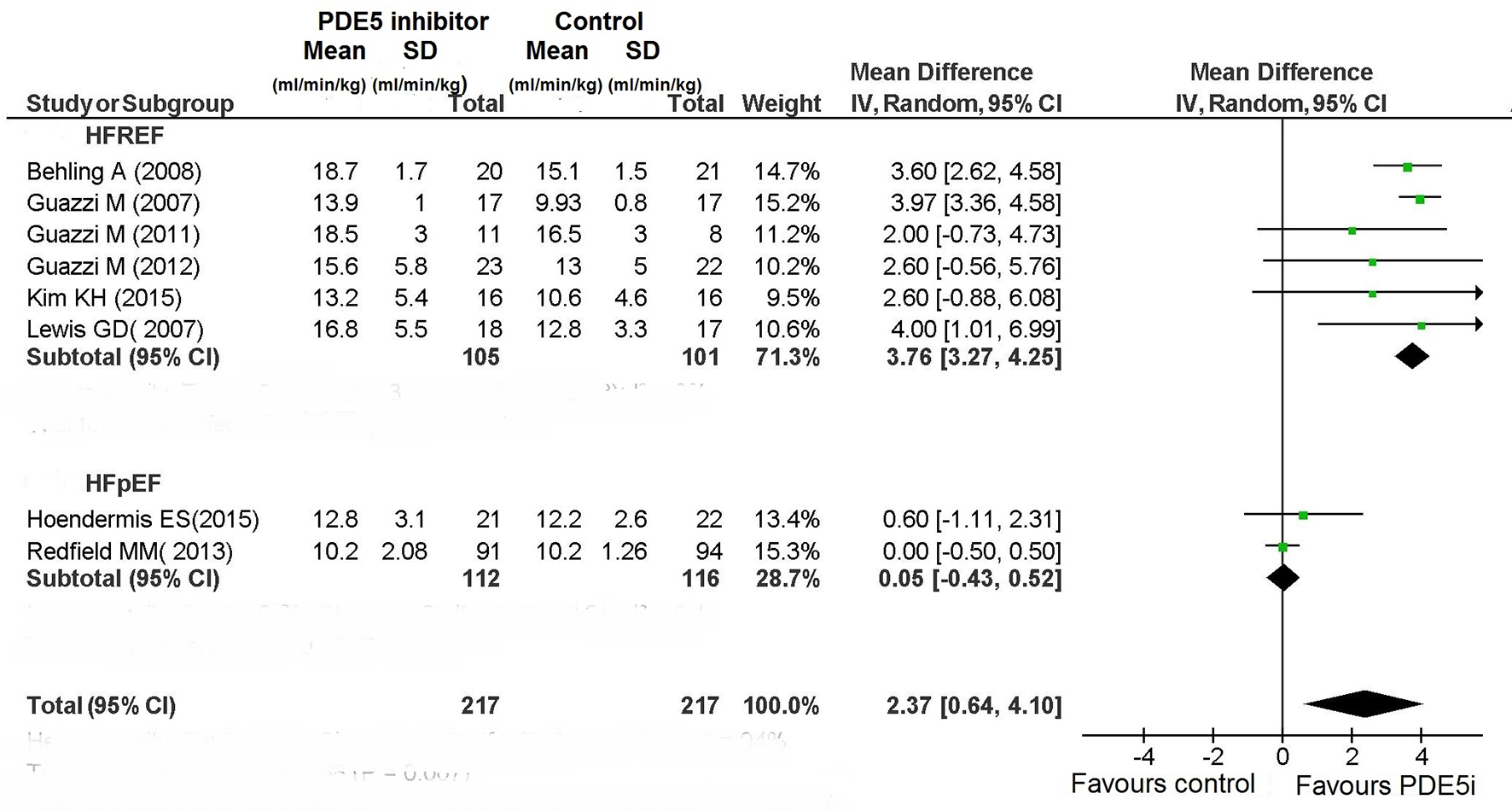 Click for large image | Figure 4. Peak VO2 in CHF. |
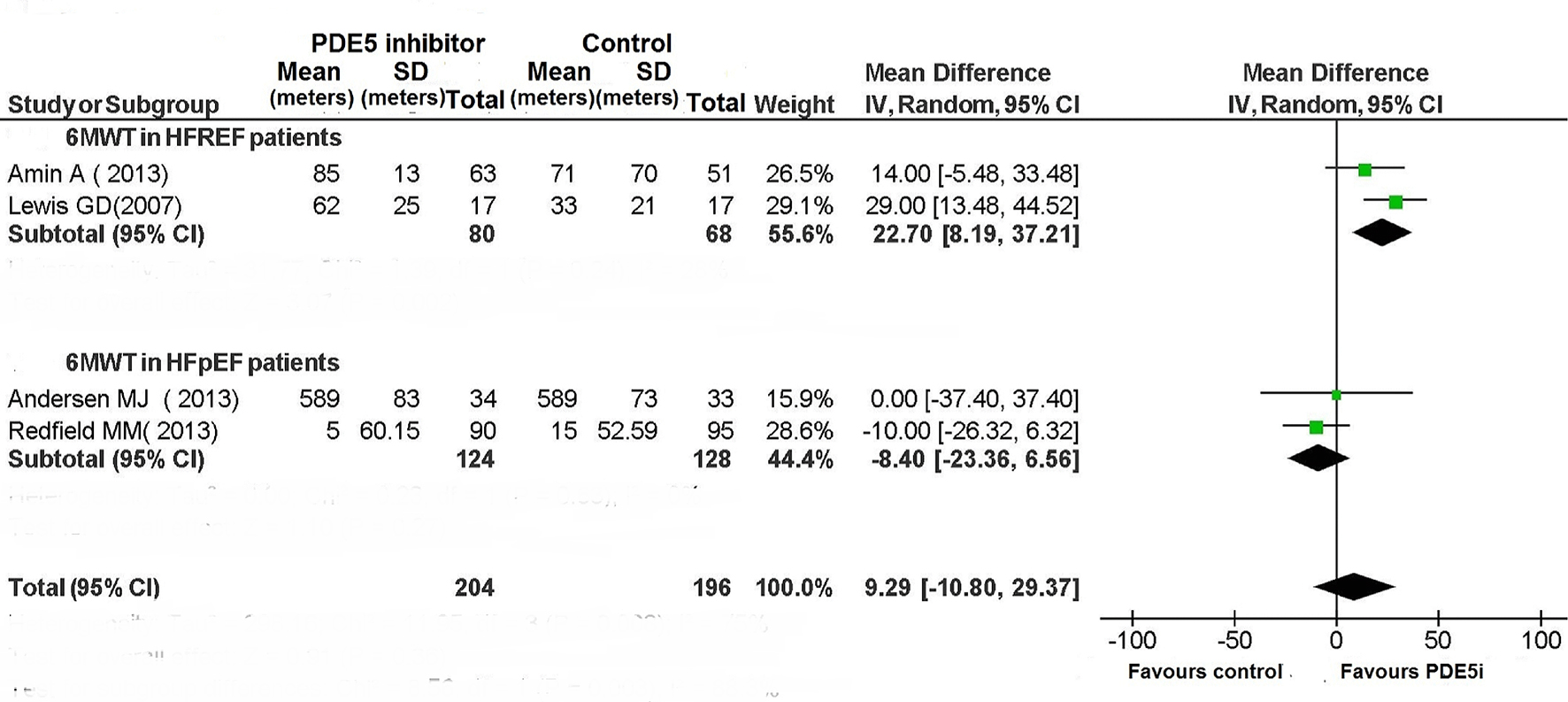 Click for large image | Figure 5. The 6MWT in patients with CHF. |
As regards the assessment of LVEF in patients with HFREF, based on the results of four studies [3, 10, 13, 16], the use of PDE5 inhibitor was associated with a significant increase in LVEF compared to placebo (MD: 4.30%; 95% CI: 2.18-6.42%; P < 0.0001; Fig. 6). By contrast, the use of PDE5 inhibitor for HFpEF patients resulted only in a non-significant tendency for increased LVEF (MD: 2.28%; 95% CI: -0.35% to 4.91%; P = 0.09; Fig. 6).
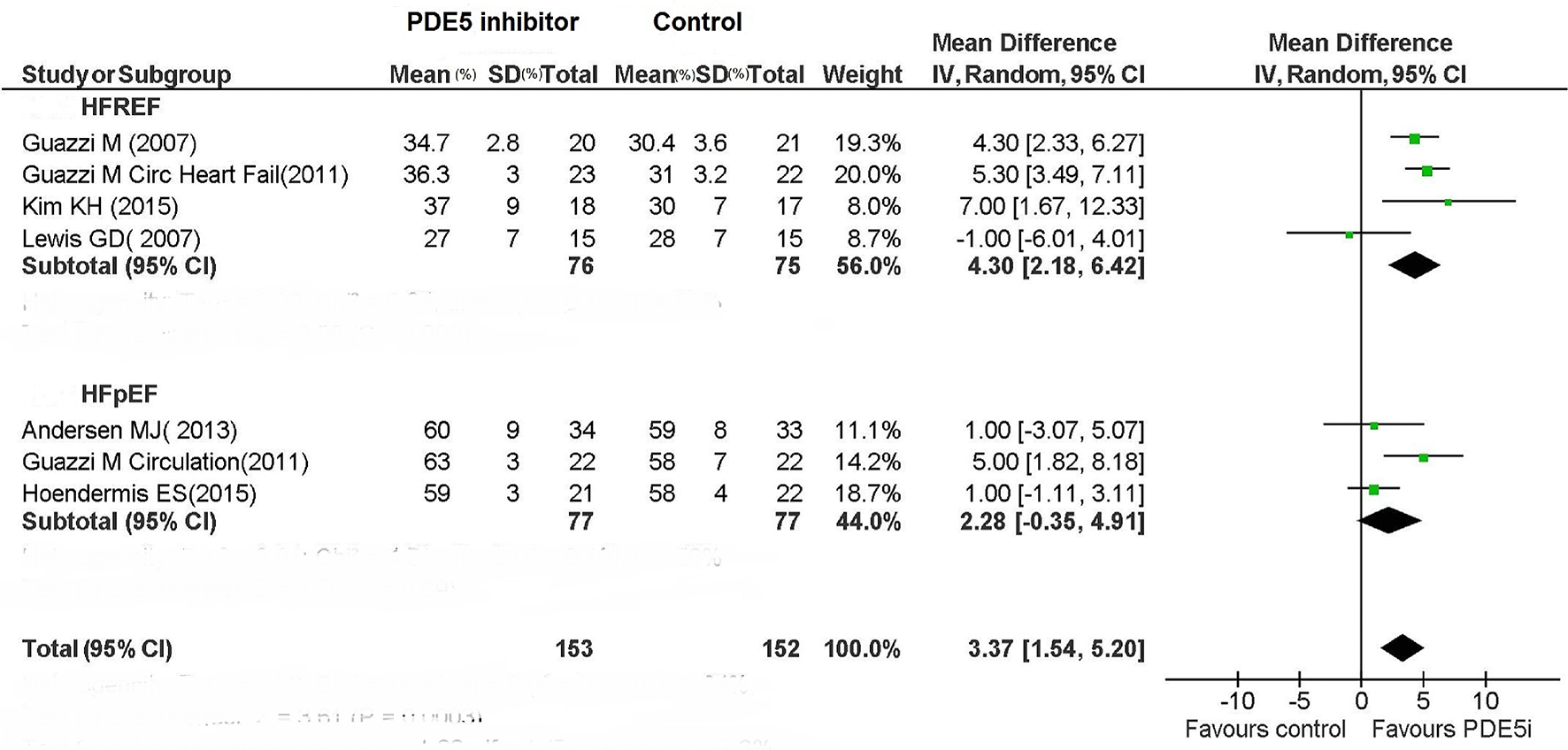 Click for large image | Figure 6. LVEF in HFREF and HFPEF patients under treatment with PDE5i. |
The use of PDE5 inhibitor in HFREF decreased mitral annular E/e’ ratio, but did not significantly affect this parameter in HFpEF (Fig. 7).
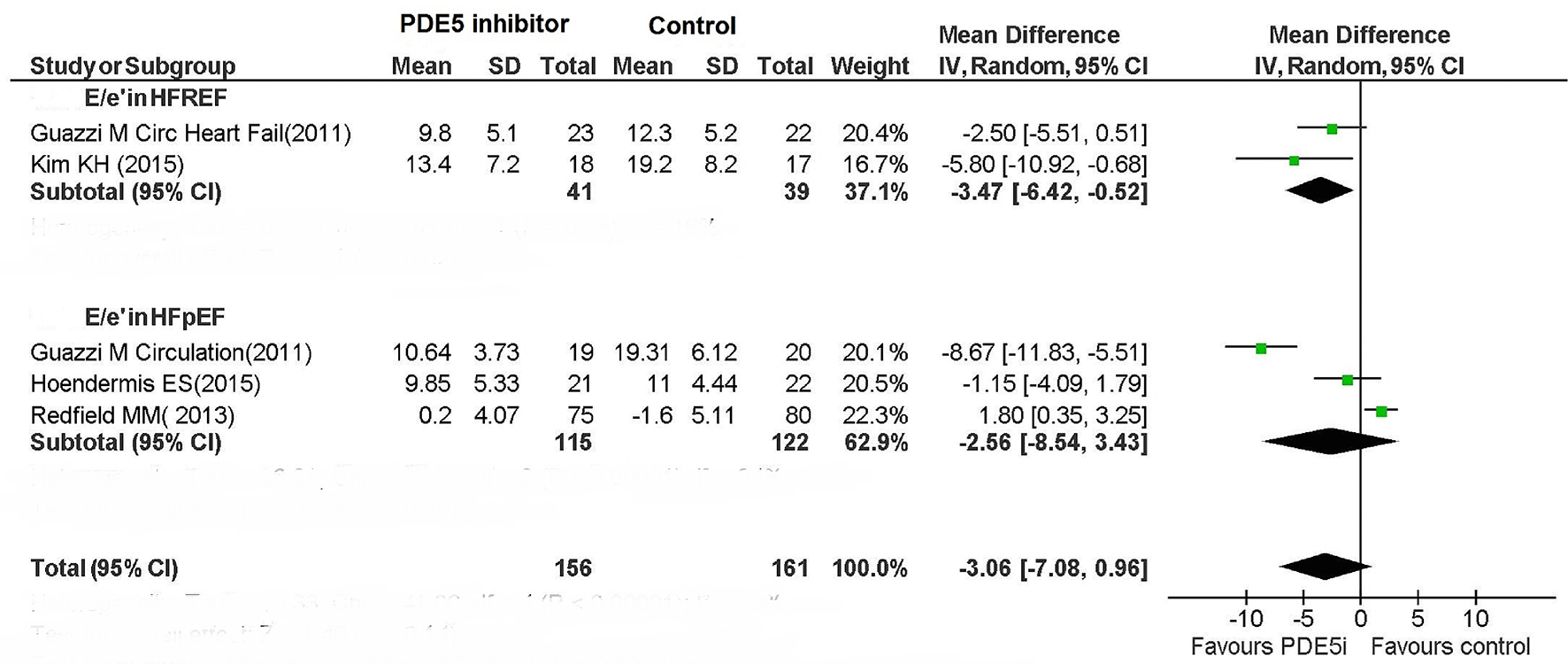 Click for large image | Figure 7. E/e’ ratio in HFREF and HFPEF patients. |
Pulmonary resistance and pulmonary pressures
For patients with HFREF, PDE5 inhibitor caused a non-significant reduction in mPAP (MD: -6.73 mm Hg; 95% CI: -14.37 to 0.91; P = 0.08), while PASP was significantly reduced (MD: -11.52 mm Hg; 95% CI: -15.56 to -7.49; P < 0.00001) (Figs. 8 and 9).
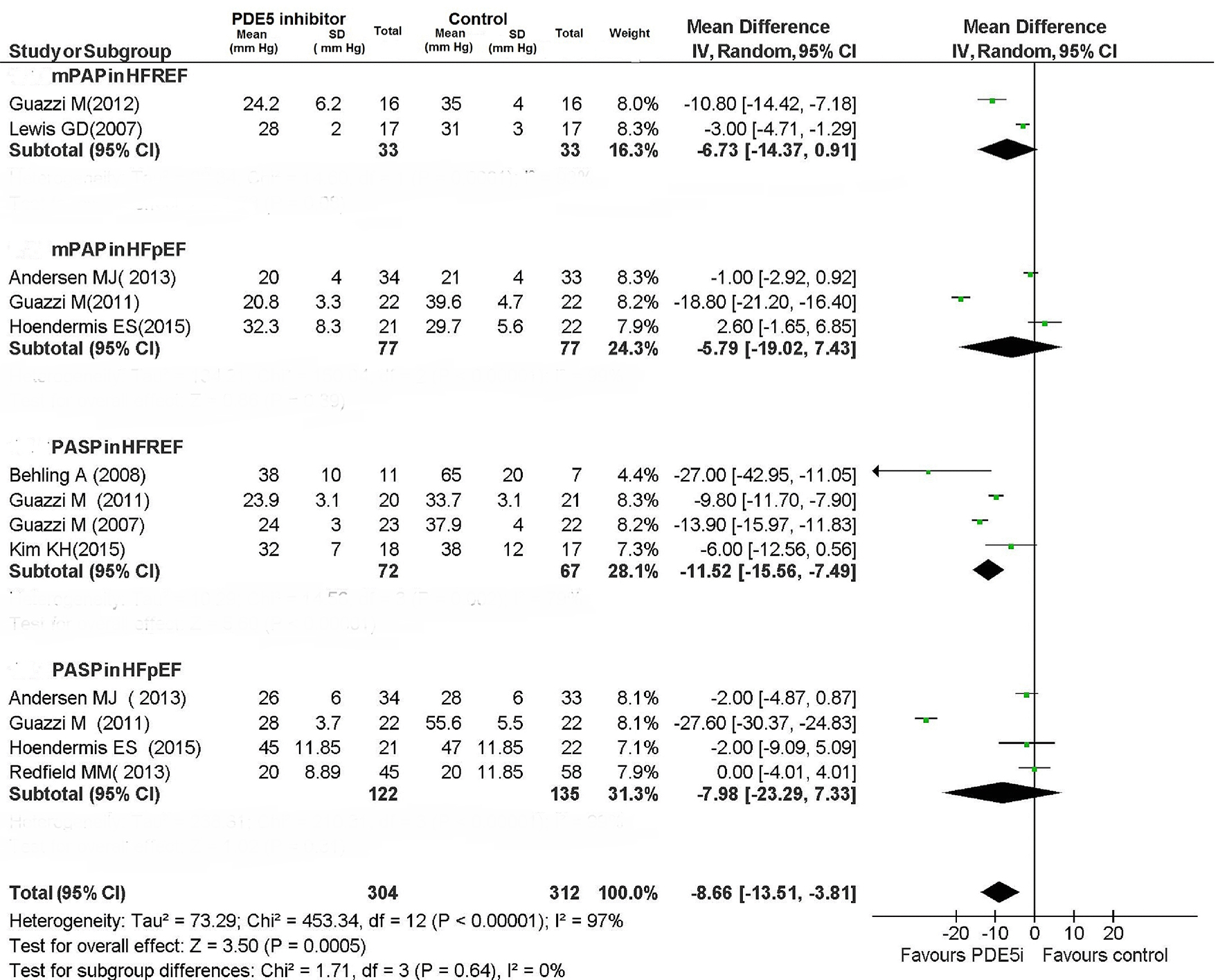 Click for large image | Figure 8. Pulmonary pressures in CHF patients. |
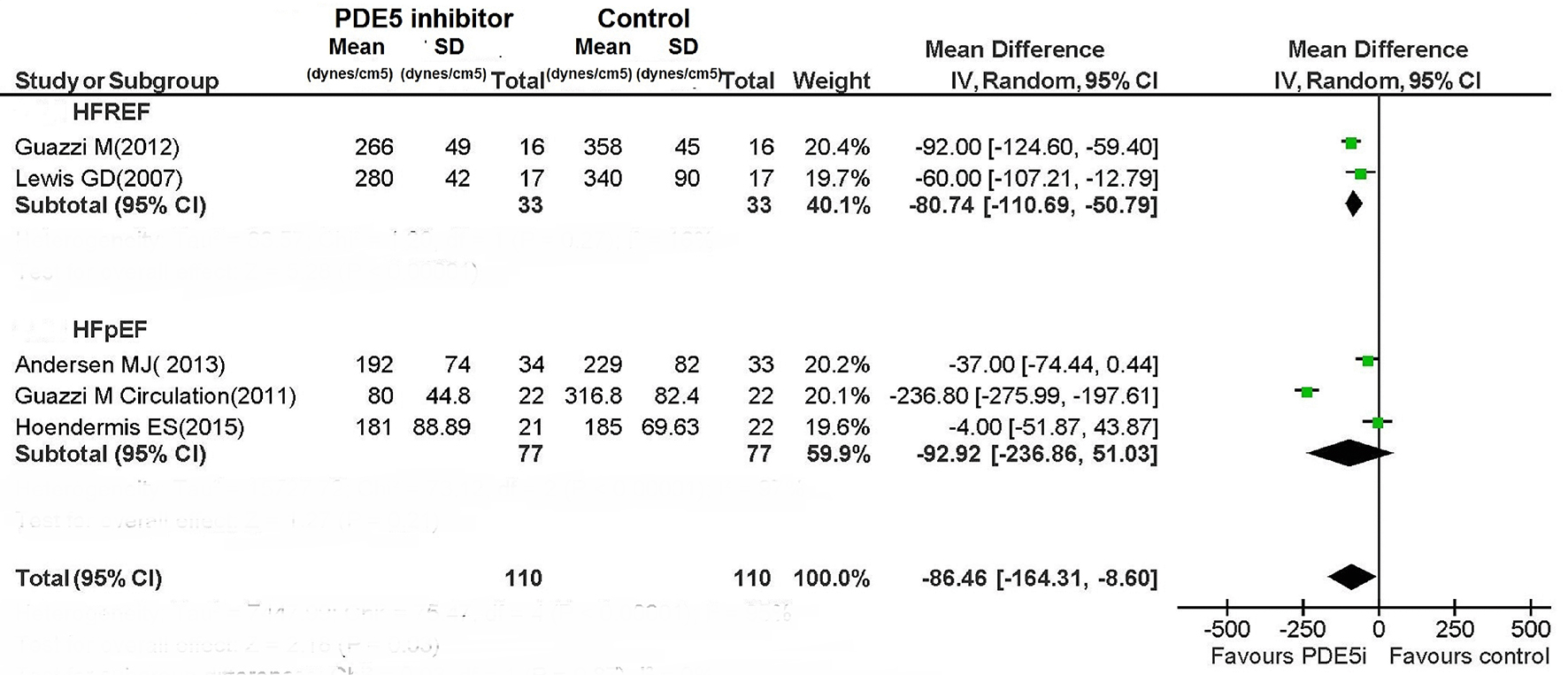 Click for large image | Figure 9. PVR during therapy with PDE5i. |
The PDE5 inhibitor-mediated improvement in pulmonary hemodynamic parameters for patients with HFREF was concordant among the RCTs. The use of PDE5 inhibitor proved not to be associated with any significant improvement in pulmonary hemodynamics in patients with HFpEF (Figs. 8 and 9); however, the included RCTs showed very high heterogeneity (Fig. 8; I2: 99% for both mPAP and PASP in HFpEF patients).
| Discussion | ▴Top |
The illustration of the various studies centered around the PDE5 inhibitor use in heart failure is far from simple. In addition, in order to explain the substantial failure of PDE5 inhibitors in HFpEF, you may need to refer to specific categories of hemodynamic profile regarding the pulmonary circulation. However, such an approach is only applicable to RCTs in which pulmonary catheterization was performed (five out 13; Tables 1 and 2).
Some aspects of this issue are highlighted below.
Favorable effects of PDE5 inhibitors in the subset of HFREF patients
First, the PDE5 inhibitors have proven to improve the composite of death and hospitalizations compared to placebo in HFREF patients. This has to be emphasized because based on seven studies [3, 7, 12, 13, 15, 16, 19], it testifies the existence of an important protective role of PDE5 inhibitors against the risk of death and hospitalizations in HFREF patients. Among the studies incorporated in the meta-analysis, sildenafil was used in six studies and udenafil in one, with a total of 460 patients investigated about the endpoint “death and hospitalizations” (Fig. 2). It should be noted that a significant effect on this “hard” endpoint was not achieved by any of the individual studies considered (Notably, two studies were not evaluable for the absence of events, i.e., lack of death or hospitalization both in the arm of PDE5 inhibitor-treated patients and in the one of controls). Therefore, a statistically significant protective effect against death and/or hospitalizations (OR: 0.28; 95% CI: 0.10 - 0.74) was inferred in HFREF patients exclusively on the basis of the overall analysis of the aggregate data. However, this result has to be reported with the due emphasis because it is a novelty, and because it helps us to propose with the due caution the PDE5 inhibitors, in particular sildenafil, as candidate drugs ready to be inserted into the group of drugs (ACE inhibitors, beta blockers, and aldosterone receptor antagonists) that on the basis of substantial clinical evidence are currently regarded capable of providing significant benefit to patients with HFREF in terms of increased survival and/or survival free from hospitalizations. Obviously further studies, again in the form of RCTs, are warranted to corroborate and validate the results of this meta-analysis. As regards the functional parameters (exercise capacity and cardiac performance), a very important and solid evidence in favor of the use of PDE5 inhibitors has emerged from our meta-analysis. Indeed a functional betterment, ensuing from the administration of PDE5 inhibitor has been documented for the exercise capacity in HFREF patients. Indeed, based on six RCTs [3, 9, 10, 12, 13, 16] with a total of 206 HFREF patients randomized to PDE5 inhibitor or placebo, a substantial improvement in the peak VO2 has been proven in the PDE5 inhibitor-treated patients. In particular, three studies have evidenced a significant increase in peak VO2. Moreover, the analysis of aggregated data has confirmed the existence of a statistically significant meaning of the increase in peak VO2 in the entire study population, related to the use of PDE5 inhibitor (weighted MD: 3.76; 95% CI: 3.27 - 4.25).
Among patients with HFREF, the 6MWT has been assessed only in two studies, whose overall evaluation by means of meta-analysis has evidenced an increase in functional capacity in the PDE5 inhibitor arm (Fig. 5). Even the LVEF was improved compared to placebo in HFREF patients taking therapy with sildenafil (Fig. 6).
In studies evaluating the measurements of the mPAP (two studies), PASP (four studies) and PVR (two studies), a significant reduction was consistently detected across the studies for each of these indexes in HFREF patients treated with PDE5 inhibitor compared to those taking placebo.
The functional, hemodynamic and clinical response of HFpEF patients to the PDE5 pharmacological inhibition: disappointing overall results that deserve further research
Different from the substantially favorable response of HFREF patients to PDE5 inhibitor administration, we did not observe any significant and consistent benefits conferred by PDE5 inhibitor treatment for patients with HFpEF. The reasons for this unsatisfactory response are at the moment unclear. In this regard, there are elements of significant perplexity in the fact that at least two studies [10, 16] would have documented an improvement in diastolic function index known as E/e’ ratio in patients with heart failure treated with sildenafil [10] or udenafil [16]. In addition, the molecular and biochemical pathways of sildenafil and related drugs, such as detected in experimental animals, appear to actually be compatible with the hypothesis of a favorable effect by PDE5 inhibitor on hemodynamic parameters and clinical outcomes of patients with HFpEF [20]. Conversely, with regard to the relatively low efficacy of PDE5 on hemodynamic and spiro-ergometric parameters, as well as on clinical outcomes in patients with HFpEF, as evidenced by some studies included in our meta-analysis [14, 18], this might depend on a possible predominance of the cases of Ipc-PH in these studies. This has been documented with certainty in the study by Hoendormis et al [14], in which a condition of Cpc-PH, regarded as a crucial element for the occurrence of a comprehensive and effective pharmacodynamic action of PDE5 inhibitor [5, 16] in the PH related to left heart disease, was present only in 12% of cases. The fact that the HFpEF patients investigated in these studies were to be ascribed predominantly to the Ipc-PH category might have played a crucial role in the generation of disappointing results.
Therefore, the thesis aimed to support a useful effect limited to the HFREF patients, due to an alleged lack of efficacy of the PDE5 inhibition in HFpEF patients should be regarded not adequately proven yet [21]. In fact, the highlighted difference about the effects reported in the two echographic phenotypes might depend on a lower frequency of Cpc-PH profile in HFpEF patients rather than on a real critical role of the type of left ventricular dysfunction (HFREF or HFpEF) in determining the clinical efficacy of the PDE5 inhibitors.
Therefore, in order to verify the possible causes of the unsatisfactory results of PDE5 ihibitors in HFpEF, further studies, conducted by recruiting HFpEF patients belonging to the Cpc-PH category, would be warranted.
Conclusions
The use of PDE5 inhibitors in patients with HFREF showed beneficial effects on pulmonary hemodynamics and exercise capacity. In addition, as regards the composite endpoint death/hospitalization, there was a significantly protective effect of PDE5 inhibitors, limited to the HFREF patients.
Conversely, the use of PDE5 inhibitors in patients with HFpEF showed disappointing results.
In fact, in the case of HFpEF patients, no significant improvement was achieved for each of the investigated endpoints (either functional, hemodynamic or clinical).
However, the hypothesis that the unfavorable results detected in HFpEF patients might have been caused by a not proper selection of the patient population (i.e., paucity of the cases of combined post- and pre-capillary PH in the studies conducted so far) should be taken into account. Thus, further studies with well-defined pulmonary hemodynamic profile, including an adequate number of HFpEF patients with Cpc-PH, would be warranted in order to better clarify the real therapeutic potential of PDE5 inhibitors even for treatment of HFpEF patients.
| References | ▴Top |
- Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975-990.
doi pubmed - Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
doi pubmed - Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555-1562.
doi pubmed - Guazzi M, Gomberg-Maitland M, Naeije R. Impact of pharmacologic interventions - treating endothelial dysfunction and group 2 pulmonary hypertension. Prog Cardiovasc Dis. 2015;57(5):473-479.
doi pubmed - Gerges M, Gerges C, Lang IM. How to define pulmonary hypertension due to left heart disease. Eur Respir J. 2016;48(2):553-555.
doi pubmed - Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W264.
- Amin A, Mahmoudi E, Navid H, Chitsazan M. Is chronic sildenafil therapy safe and clinically beneficial in patients with systolic heart failure? Congest Heart Fail. 2013;19(2):99-103.
doi pubmed - Andersen MJ, Ersboll M, Axelsson A, Gustafsson F, Hassager C, Kober L, Borlaug BA, et al. Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction: the Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction (SIDAMI) trial. Circulation. 2013;127(11):1200-1208.
doi pubmed - Behling A, Rohde LE, Colombo FC, Goldraich LA, Stein R, Clausell N. Effects of 5'-phosphodiesterase four-week long inhibition with sildenafil in patients with chronic heart failure: a double-blind, placebo-controlled clinical trial. J Card Fail. 2008;14(3):189-197.
doi pubmed - Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4(1):8-17.
doi pubmed - Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164-174.
doi pubmed - Guazzi M, Vicenzi M, Arena R. Phosphodiesterase 5 inhibition with sildenafil reverses exercise oscillatory breathing in chronic heart failure: a long-term cardiopulmonary exercise testing placebo-controlled study. Eur J Heart Fail. 2012;14(1):82-90.
doi pubmed - Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50(22):2136-2144.
doi pubmed - Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36(38):2565-2573.
doi pubmed - Katz SD, Parker JD, Glasser DB, Bank AJ, Sherman N, Wang H, Sweeney M. Efficacy and safety of sildenafil citrate in men with erectile dysfunction and chronic heart failure. Am J Cardiol. 2005;95(1):36-42.
doi pubmed - Kim KH, Kim HK, Hwang IC, Cho HJ, Je N, Kwon OM, Choi SJ, et al. PDE 5 inhibition with udenafil improves left ventricular systolic/diastolic functions and exercise capacity in patients with chronic heart failure with reduced ejection fraction; A 12-week, randomized, double-blind, placebo-controlled trial. Am Heart J. 2015;169(6):813-822 e813.
- Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1(4):227-233.
doi pubmed - Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268-1277.
doi pubmed - Webster LJ, Michelakis ED, Davis T, Archer SL. Use of sildenafil for safe improvement of erectile function and quality of life in men with New York Heart Association classes II and III congestive heart failure: a prospective, placebo-controlled, double-blind crossover trial. Arch Intern Med. 2004;164(5):514-520.
doi pubmed - Matyas C, Nemeth BT, Olah A, Torok M, Ruppert M, Kellermayer D, Barta BA, et al. Prevention of the development of heart failure with preserved ejection fraction by the phosphodiesterase-5A inhibitor vardenafil in rats with type 2 diabetes. Eur J Heart Fail. 2017;19(3):326-336.
doi pubmed - Guazzi M, van Heerebeek L, Paulus WJ. Phosphodiesterase-5 inhibition in heart failure with preserved ejection fraction: trading therapy for prevention. Eur J Heart Fail. 2017;19(3):337-339.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


