| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 9, Number 5, May 2017, pages 439-445
Cardio-Ankle Vascular Index and C-Reactive Protein Are Useful Parameters for Identification of Ischemic Heart Disease in Acute Heart Failure Patients
Shunsuke Kiuchia, b, Shinji Hisatakea, Takayuki Kabukia, Takashi Okaa, Shintaro Dobashia, Takahiro Fujiia, Takanori Ikedaa
aDepartment of Cardiovascular Medicine, Toho University Graduate School of Medicine, Tokyo, Japan
bCorresponding Author: Shunsuke Kiuchi, Department of Cardiovascular Medicine, Toho University Graduate School of Medicine, 6-11-1 Omorinishi, Ota-ku, Tokyo 143-8541, Japan
Manuscript accepted for publication March 09, 2017
Short title: CAVI and CRP in Patients With Acute HF
doi: https://doi.org/10.14740/jocmr2994w
| Abstract | ▴Top |
Background: The most common cause of heart failure (HF) is ischemic heart disease (IHD). Evaluation of IHD with non-invasive examinations is useful for the treatment of HF, and cardio-ankle vascular index (CAVI) is a good parameter for detecting systemic arteriosclerosis. However, the relationship between IHD and CAVI in acute HF (AHF) patients is still unclear. Therefore, we investigated the effect of non-invasive examinations, including CAVI to detect IHD.
Methods: We studied 53 consecutive patients (average age of 66.5 ± 10.9 years old, 36 males) with AHF from January 2009 to December 2012. These patients were classified into the IHD group (n = 19) and non-IHD group (n = 34) according to the coronary artery angiography results. We evaluated the vital signs, laboratory findings and CAVI.
Results: According to the laboratory findings, the C-reactive protein (CRP) in IHD group was significantly higher than non-IHD group (1.5 ± 2.1 mg/dL vs. 0.4 ± 0.4 mg/dL, P = 0.002). CAVI in IHD group was significantly higher than non-IHD group (9.58 ± 1.73 vs. 7.83 ± 1.86, P < 0.001). In the receiver operating characteristic (ROC) curve for discriminating the probability of IHD, the cut-off point of the CRP plus CAVI was 9.00. At that cut-off point, the sensitivity and the specificity were 69.7% and 89.5%, respectively. The mean area under the ROC curve (AUC) defined by the CRP plus CAVI was the greatest at all parameters.
Conclusion: The CRP and CAVI were useful parameters for the identification of IHD in patients with AHF.
Keywords: Cardio-ankle vascular index; C-reactive protein; Heart failure; Ischemic heart disease
| Introduction | ▴Top |
Acute heart failure (AHF) is a leading cause of morbidity and mortality in industrialized countries [1]. Therefore, the diagnosis, prevention and treatment of AHF are important. Treatment of heart failure (HF) differs with the presence of ischemic heart disease (IHD). We diagnose IHD using coronary artery angiography (CAG), multi-detector row computed tomography (MDCT) or nuclear cardiology test. These tests need to be performed after the improvement of AHF, because contrast media is necessary in CAG and MDCT, and nuclear cardiology test involves drug or exercise stress. Non-invasive examinations for IHD evaluation could result in effective treatments for HF.
In addition, cardio-ankle vascular index (CAVI), which is less influenced by blood pressure (BP), is a useful parameter for detecting arteriosclerosis [2]. CAVI can be calculated from pulse wave velocity (PWV) at the origin of the aorta to the ankle portion of the tibial artery, and systolic and diastolic BPs (sBPs and dBPs) measured at the upper brachial artery. This index was originally derived from stiffness parameter β. Previous studies revealed the relationship between CAVI and IHD [3, 4]. This study also shows that CAVI was significantly higher in 1 vessel disease (VD) group compared to the 0 VD group (P < 0.05), and was significantly higher in the 2 VD and 3 VD groups compared to the 1 VD group. However, the relationship between IHD and CAVI in HF patients is still unclear. The aim of this study was to investigate its effectiveness of non-invasive examinations, including CAVI, to detect IHD in AHF patients.
| Materials and Methods | ▴Top |
All experiments were performed in accordance with the Declaration of Helsinki and were approved by the Toho University Omori Medical Center Ethical Committee (25-193).
Study populations
We studied 329 consecutive patients hospitalized for AHF from January 2009 to December 2012 at Toho University Omori Medical Center. The inclusion criteria were 1) hospitalization due to AHF at first time, and 2) patients have undergone ankle-brachial index (ABI) and CAG within hospitalization. The exclusion criteria were as follows: 1) age: under 29 years and over 85 years, 2) chronic HF patients with acute exacerbation, 3) serum troponin I (TnI) over 2.0, 4) hemodialysis patients, 5) past history of vascular disease, 6) past history of cardiovascular operations and/or cardiovascular catheter examinations, and 7) ABI under 0.9 and over 1.3. Finally, 53 patients became subject of this study and were divided into IHD group (n = 19) and non-IHD group (n = 34) by CAG.
CAG
We analyzed coronary artery using CAG after the improvement of HF. Visual assessment of the stenotic lesion was undertaken based on the American Heart Association classification. The percentage ratio of the stenotic lumen to the original vessel diameter of the lesion analogized by a presumed-to-be-healthy site distal and proximal to the stenosis was obtained and the degree of stenosis was expressed by subtracting this from 100. Out of end-diastolic still images taken from multiple projections and measurements were taken in the angle showing the greatest degree of stenosis to classify the lesion into six stages: 25% for stenosis of 0-25%, 50% for 26-50%, 75% for 51-75%, 90% for 76-90%, 99% for 91-99%, and 100% for total occlusions. Lesions with stenosis of 75% or more were defined to be significant stenotic lesions and were divided into IHD group.
Clinical profile
Age, sex (male percentage), sBP and dBP, and heart rate (HR) were evaluated. BP and HR were measured at the time of hospitalization. And, we also analyzed New York Heart Association classification (NHYA).
Laboratory analysis
We measured lipid metabolism, glycemic metabolism, liver and renal functions, myocardial damage markers, brain natriuretic peptide (BNP) and C-reactive protein (CRP). Total cholesterol (T-Cho), triglyceride (TG) and high-density lipoprotein-cholesterol (HDL) were measured as lipid metabolism. Low-density lipoprotein-cholesterol (LDL) and non-HDL were calculated with the formula: LDL = T-Cho - HDL - TG/5 and non-HDL = T-Cho - HDL, respectively [5, 6]. Fasting blood glucose (FBS) and hemoglobin A1C (HbA1C) were measured as glycemic metabolism. We evaluated liver functions by albumin, total-bilirubin, aspartate transaminase, aspartate aminotransferase and lactate dehydrogenase. Renal functions were evaluated by blood urea nitrogen and creatinine. We measured creatine kinase (CK), CK muscle and brain (CK-MB) and TnI as myocardial damage markers. All serum samples were obtained at the time of hospitalization.
Electrocardiography (ECG) and transthoracic echocardiography (TTE)
We analyzed standard 12-lead ECG at hospitalization. Analysis of the ECG findings was performed with visual inspection of two cardiologists. Ischemic change of ECG was defined as: 1) down-slope or horizontal ST depressions, 2) ST elevations, 3) T wave abnormalities, including negative T wave, and 4) abnormal Q wave and poor R progression [7]. And, we also checked heart rhythms.
TTE was investigated within 2 days of admission. Left ventricular (LV) systolic function was evaluated with ejection fraction from the Teichholz method [8], or modified Simpson’s method [9]. Visual regional wall motion was interpreted according to the American Society of Echocardiography criteria with a 17-segment model [10]. Regional myocardial contractile function was graded as normal, hypokinesis, akinesis, or dyskinesis for each myocardial segment. Regional wall motion abnormalities were evaluated with two cardiologists.
CAVI
CAVI and ABI were measured according to the methods described previously, using a VaSera VS-1500E manufactured by Fukuda Denshi Company, Ltd (Tokyo, Japan) [11]. CAVI and ABI were evaluated after the improvement of HF and measured in the morning after 12 h of fasting. Their electrocardiogram and heart sounds were monitored after the patients kept in the dorsal position comfortably for at least 10 min. Cuffs were applied to the bilateral upper arms and ankles. PWV was obtained by dividing vascular length by the time taken for the pulse wave to propagate from the aortic valve to the ankle. The formula used to calculate CAVI was as follows: CAVI = a((2ρ × 1/(sBP - dBP)) × (In(sBP/dBP) × PWV2)) + b, where ρ is blood density, and a and b are constants to match aortic PWV [11]. We calculated the average of right and left CAVI.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. We compared between both groups by unpaired Student’s t-test. Statistical significance was considered at P < 0.05 in all instances. We investigated the receiver operating characteristic (ROC) curve, which is defined as a plot of sensitivity versus its 1-specificity or false positive rate. The ROC was analyzed with a Windows computer (Excel (Microsoft XP)) and EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 2.13.0) [12].
| Results | ▴Top |
Clinical profiles
The clinical profile of the patients is summarized in Table 1. There were also no differences in average age and gender. In addition, there were also no significant differences of sBP, dBP and HR. The prevalence of diabetes mellitus (DM) in IHD group was significantly higher than in non-IHD group. DM was diagnosed with administration of glucose lowering medications or diagnostic criteria of the Japanese Diabetes Society. Most of the patients, who have DM or other life style related diseases, were treated with diet and/or exercise, therefore, no significant differences were shown in the use of BP, lipid and glucose lowering drugs between both groups.
 Click to view | Table 1. Clinical Profiles Between Both Groups |
Laboratory findings between both groups
In laboratory findings, no significant differences were shown in liver and renal functions (Table 2). In addition, there were also no differences in lipid metabolism. There were no significant differences, although HbA1C in IHD group was higher than in non-IHD group. At the time of hospitalization, no significant difference was seen in the levels of HF evidenced with NYHA and BNP between both groups. Moreover, no difference was indicated in myocardial damage markers either. On the other hand, CRP in IHD group was significantly higher than in non-IHD group (1.5 ± 2.1 mg/dL vs. 0.4 ± 0.4 mg/dL, P < 0.001, Table 2). We investigated the correlation between CRP and IHD. By using the ROC curve, we investigated the possibility for CRP to detect IHD. The cut-off point that gave the maximal sensitivity and specificity for CRP was 1.7 (Fig. 1a). The sensitivity and the specificity for CRP were 97.0% and 36.8%, respectively. AUC and 95% confidence interval (CI) were 0.625 and 0.461 - 0.79, respectively.
 Click to view | Table 2. Laboratory Findings Between Both Groups |
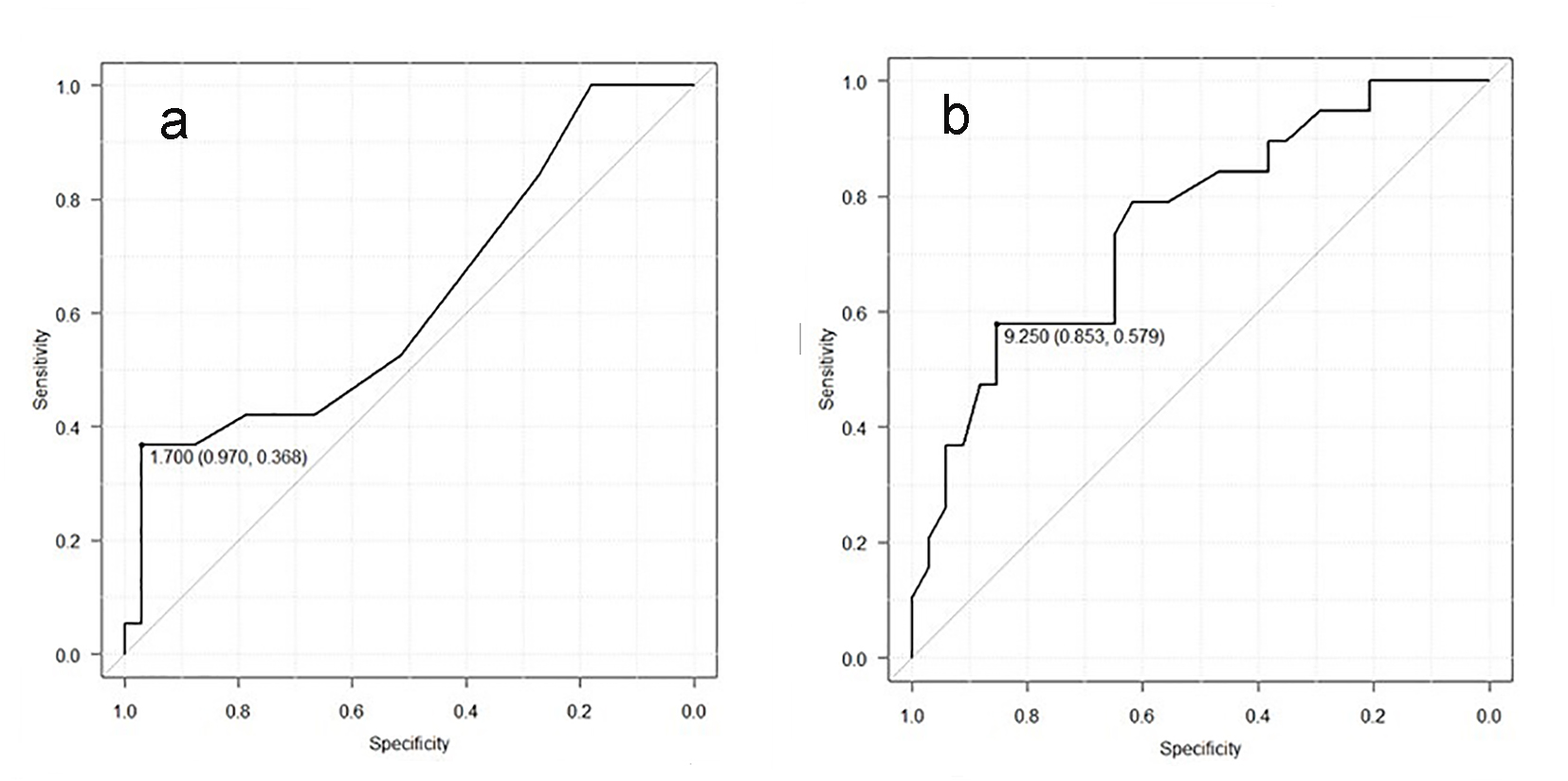 Click for large image | Figure 1. (a) Receiver operating curve of CRP for detection of the IHD group. The cut-point value was 1.7. AUC and 95% CI were 0.625 and 0.461 - 0.79, respectively. (b) Receiver operating curve of CAVI for detection of the IHD group. The cut-point value was 9.25. AUC and 95% CI were 0.752 and 0.615 - 0.89, respectively. |
Heart structure with ECG, TTE and CAVI between both groups
Five patients have atrial fibrillation (AF) and/or atrial flutter (AFL) in IHD group, while eight patients have AF and/or AFL in non-IHD group. All patients did not have ST elevation and abnormal Q wave in this study. Other ischemic changes of ECG were shown in two and three patients of IHD and non-IHD group. These ECG findings showed no significant differences between both groups. There were also no significant differences in TTE parameters (Table 3). Regional wall motion abnormalities were found in five and zero patients of IHD and non-IHD group, respectively. CAVI in IHD group was significantly higher than in non-IHD group (9.58 ± 1.73 vs. 7.83 ± 1.86, P < 0.001), although there were no differences in ABI between both groups (Table 3). We investigated the possibility for CAVI to detect IHD, using ROC curve. The cut-off point that gave the maximal sensitivity and specificity for CAVI was 9.25 (Fig. 1b). The sensitivity and the specificity for CAVI were 85.3% and 57.9%, respectively. AUC and 95% CI were 0.752 and 0.615 - 0.89, respectively.
 Click to view | Table 3. CAVI and Echocardiographic Findings Between Both Groups |
The ROC area defined the CRP plus CAVI
We also investigated the CRP plus CAVI, because of the low specificity for CAVI. The CRP plus CAVI in IHD group was significantly higher than in non-IHD group (11.09 ± 2.45 vs. 8.20 ± 1.85, P < 0.001, Fig. 2a). The sensitivity and the specificity for the CRP plus CAVI were 69.7% and 89.5% at the cut-off point for the CRP plus CAVI (9.00) (Fig. 2b). AUC was 0.839, which was the greatest among all parameters. 95% CI was 0.719 - 0.959. AUC of the CRP plus CAVI was significantly higher than that of CRP and CAVI (P = 0.033 and P = 0.042, respectively).
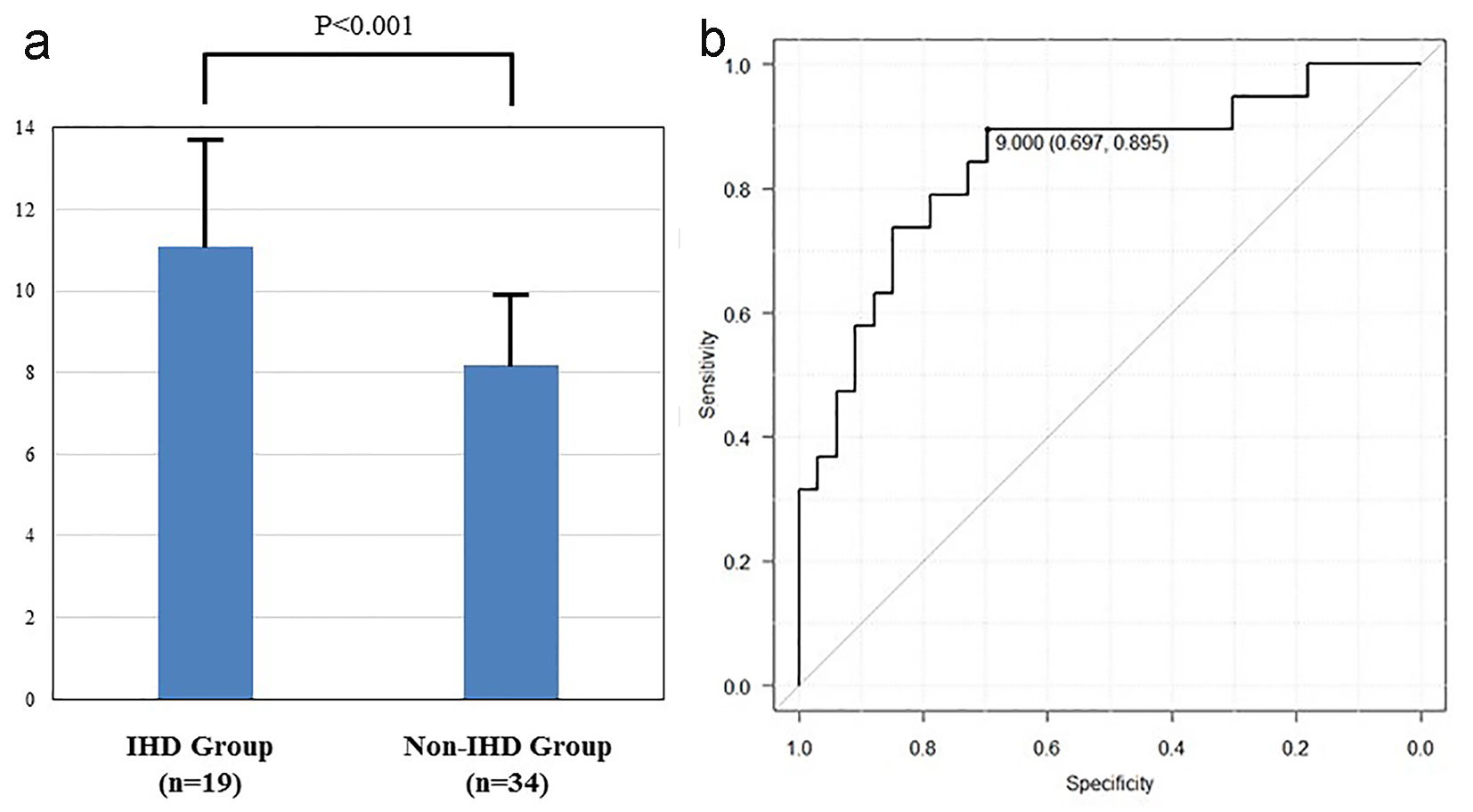 Click for large image | Figure 2. (a) The CRP plus CAVI in IHD group was significantly higher than in non-IHD group (11.09 ± 2.45 vs. 8.20 ± 1.85, P < 0.001). (b) Receiver operating curve of CAVI plus CRP for detection of the IHD group. The cut-point value was 9.00. AUC and 95% CI were 0.839 and 0.719 - 0.959, respectively. |
| Discussion | ▴Top |
Main findings
Previous study reported that all-cause mortality rate and hospitalization rate at 1 year in AHF patients were 17.4% and 43.9%, respectively [13]. The most common cause of AHF in Japan is IHD, although cause of AHF varies [14]. Vasodilator needs to be used for AHF patients with IHD, and BPs need to be maintained carefully, because coronary flow is defined in BPs. Moreover, AHF due to ischemia increases risk of cardiac death [15]. Therefore, identification of IHD is vital for AHF patients. In this study, we could not determine IHD by only ECG and/or UCG. However, we were able to determine IHD by the CRP plus CAVI. If CAG cannot be performed in AHF patients, the CRP plus CAVI is a useful parameter for detecting IHD.
CRP and CAVI in IHD
It has been reported that elevated CRP is a predictor of adverse cardiovascular events [16], because atherosclerosis is caused by vascular chronic inflammation [17]. CRP is a good indicator of the diagnosis of atherosclerosis. CRP (as a complex parameter) is correlated with chronic inflammation, although individual parameters such as glucose and lipid metabolism disorder are only slightly related to chronic inflammation. Therefore, only CRP might show significant difference in the laboratory findings in this study. Other coronary risk factors in laboratory findings had no significant differences. It has also been reported that CRP is a better parameter to predict IHD than LDL [18]. However, it is not enough to evaluate IHD only by using CRP, because other inflammation can also include vascular chronic inflammation and systemic inflammation, such as cold.
The relationship between CAVI and arteriosclerosis has also been reported [19], and CAVI is known to be associated with various types of arteriosclerotic parameters [20]. Arteriosclerosis has been widely understood as a chronic inflammatory disease [21]. Leukocyte infiltration results from various factors, like oxidized LDL. Arteriosclerosis induces chronic inflammation through the inflammatory cytokine [22]. In this study, we were able to find CAVI increased in IHD group due to chronic inflammation. It has already been reported that CAVI is correlated with CRP [23]. However, a correlation between CAVI and CRP was not indicated (data not shown) due to the fact that CRP indicated other systemic inflammation as well. Therefore, the use of the CRP plus CAVI resulted in the most useful parameter to detect IHD in this study. It is possible to add CAVI to CRP to exclude systemic inflammation.
CAVI and HF
Previous studies have also reported that chronic inflammation is related to the worsening of HF and induced inflammatory cytokine worsen HF [24-26]. Furthermore, CRP is related to the prognosis of HF [27]. Thus, CAVI might be associated with HF, through chronic inflammation. Moreover, HMG-CoA reductase inhibitor (statin), as inflammation reducing medication, reduced cardiovascular events for HF patients [28]. It is possible that CAVI predicts the prognosis of HF. In this study, CAVI in non-IHD group was 8.20 ± 1.85, which is almost equivalent to the reference value of CAVI in Japan.
Study limitations
This retrospective study has several limitations. These various examinations were measured in only a small number of AHF patients. A larger number of patients with different coronary risk factors such as hypertension, dyslipidemia, DM, and smoking may reveal significant differences between both groups. In addition, CAVI in non-IHD group may be higher than the reference value of CAVI in Japan. Thus, the relationship with CAVI and HF remains unknown. The result in this study might be affected by the fact that CAVI was evaluated only after the improvement of HF. In addition, not all AHF patients received CAVI and CAG during the study period. Further clinical trials with larger continuous population are needed to investigate the relationship between these coronary risk factors, CAVI and CAG.
Conclusions
This small-scale retrospective study demonstrates that the CRP and CAVI are the most useful parameters for identification of IHD. However, the relationship between the CRP and CAVI and the mortality of the HF patients are still unclear. In order to clarify the relationship between HF, CAVI and CRP, large-scale clinical studies are required.
Disclosures
TI received research funds and lecture fees from Mitsubishi Tanabe Pharma Co., Ltd, Daiichi-Sankyo, Co., Ltd, and Ono Pharmaceutical, Co., Ltd. The remaining authors declare that there are no conflicts of interest. The authors have no financial conflicts of interest to disclose concerning this paper.
| References | ▴Top |
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007-2018.
doi pubmed - Kubozono T, Miyata M, Ueyama K, Nagaki A, Otsuji Y, Kusano K, Kubozono O, et al. Clinical significance and reproducibility of new arterial distensibility index. Circ J. 2007;71(1):89-94.
doi pubmed - Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72(4):598-604.
doi pubmed - Sairaku A, Eno S, Hondo T, Teragawa H, Nakano Y, Matsuda K, Kisaka T, et al. Head-to-head comparison of the cardio-ankle vascular index between patients with acute coronary syndrome and stable angina pectoris. Hypertens Res. 2010;33(11):1162-1166.
doi pubmed - Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502.
pubmed - Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101(7):1003-1008.
doi pubmed - Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002;106(14):1883-1892.
doi pubmed - Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37(1):7-11.
doi - Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358-367.
doi - Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463.
doi pubmed - Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101-107.
doi pubmed - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed - Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808-817.
doi pubmed - Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Goto D, Yamada S, Yokoshiki H, Takeshita A, et al. Loop diuretic use at discharge is associated with adverse outcomes in hospitalized patients with heart failure: a report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD). Circ J. 2012;76(8):1920-1927.
doi pubmed - Hamaguchi S, Kinugawa S, Goto D, Tsuchihashi-Makaya M, Yokota T, Yamada S, Yokoshiki H, et al. Predictors of long-term adverse outcomes in elderly patients over 80 years hospitalized with heart failure. - A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2011;75(10):2403-2410.
doi pubmed - Park HE, Cho GY, Chun EJ, Choi SI, Lee SP, Kim HK, Youn TJ, et al. Can C-reactive protein predict cardiovascular events in asymptomatic patients? Analysis based on plaque characterization. Atherosclerosis. 2012;224(1):201-207.
doi pubmed - Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868-874.
doi pubmed - Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557-1565.
doi pubmed - Kadota K, Takamura N, Aoyagi K, Yamasaki H, Usa T, Nakazato M, Maeda T, et al. Availability of cardio-ankle vascular index (CAVI) as a screening tool for atherosclerosis. Circ J. 2008;72(2):304-308.
doi pubmed - Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, et al. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J. 2007;71(11):1710-1714.
doi pubmed - Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74(2):213-220.
doi pubmed - Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100(8):4736-4741.
doi pubmed - Higashiyama A, Wakabayashi I, Kubota Y, Adachi Y, Hayashibe A, Nishimura K, Sugiyama D, et al. Does high-sensitivity C-reactive protein or low-density lipoprotein cholesterol show a stronger relationship with the cardio-ankle vascular index in healthy community dwellers?: the KOBE study. J Atheroscler Thromb. 2012;19(11):1027-1034.
doi pubmed - Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev. 2010;15(6):543-562.
doi pubmed - Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118(6):625-631.
doi pubmed - Miettinen KH, Lassus J, Harjola VP, Siirila-Waris K, Melin J, Punnonen KR, Nieminen MS, et al. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10(4):396-403.
doi pubmed - Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, Jasper S, et al. Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol. 2008;101(3):370-373.
doi pubmed - Lorgelly PK, Briggs AH, Wedel H, Dunselman P, Hjalmarson A, Kjekshus J, Waagstein F, et al. An economic evaluation of rosuvastatin treatment in systolic heart failure: evidence from the CORONA trial. Eur J Heart Fail. 2010;12(1):66-74.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


