| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 8, Number 12, December 2016, pages 921-924
Fulminant Hepatic Failure Secondary to Herpes Hepatitis in a Patient With Myasthenia Crisis: An Elusive Diagnosis
Harish Patela, Hassan Tariqa, b, Vinaya Gaduputia, Trupti Vakdea, Jasbir Makkera, Myrta Daniela
aDepartment of Medicine, Bronx Lebanon Hospital Center, 1650 Selwyn Ave, Suite #10C, Bronx, NY 10457, USA
bCorresponding Author: Hassan Tariq, Department of Medicine, Bronx Lebanon Hospital Center, 1650 Selwyn Ave, Suite #10C, Bronx, NY 10457, USA
Manuscript accepted for publication September 28, 2016
Short title: Fulminant Hepatic Failure
doi: http://dx.doi.org/10.14740/jocmr2701w
| Abstract | ▴Top |
Herpes hepatitis is a rare cause of fulminant hepatic failure contributing to less than 1% of all cases. It is most often seen in persons who are immunosuppressed and in pregnant women. The presentation is usually non-specific and rapidly progressive, thus making antemortem diagnosis of this condition rare. We present a patient who was on chronic immunosuppressive therapy for systemic lupus erythematosus and subsequently developed multi-organ failure with anicteric transaminitis as a result of disseminated herpes infection. The diagnosis was only made post-mortem. A confounding factor in this case was the fact that the patient underwent plasmapheresis, which skewed the interpretation of liver function tests in the setting of acute liver failure.
Keywords: Herpes hepatitis; Anicteric hepatitis; Plasmapheresis; Transaminitis; Hepatic failure; Plasmapheresis and liver function tests
| Introduction | ▴Top |
Herpes hepatitis is a well-known, albeit rare cause of fulminant hepatic failure [1]. The case fatality rate of herpetic hepatitis is quite high (41-79%) partly due to its atypical presentation and rapid progression [2]. The presenting signs and symptoms are often non-specific, including fever, abdominal pain, coagulopathy and transaminitis [3, 4] in patients who are pregnant or immunosuppressed [3, 5]. In spite of the fact that biopsy can yield a definite diagnosis [6], this condition remains grossly under-diagnosed antemortem. Prompt initiation of acyclovir therapy has been known to significantly reduce the morbidity and mortality [6]. Hence, it is important to make physicians aware of this cause of fulminant hepatic failure. We report a case of a young woman who was immunosuppressed and ultimately developed disseminated herpes with fulminant hepatic failure resulting in her death. Physicians should be careful while interpreting the liver function tests in patients undergoing plasmapheresis, which can skew the interpretation of liver function tests in the setting of acute liver failure.
| Case Report | ▴Top |
We present a case of a 22-year-old African American female who presented to the emergency room with worsening respiratory distress and drooping of eyelids for the past 2 weeks. The patient had a protracted history of systemic lupus erythematosus (SLE) diagnosed at the age of 11 with resultant lupus nephritis that was initially treated with cyclophosphamide and mycophenolate, and later replaced with azathioprine and prednisone at the time of her pregnancy. Her medical history was also significant for hypothyroidism, hypertension and myasthenia gravis.
She was admitted to the intensive care unit with acute hypoxic respiratory failure requiring endotracheal intubation and mechanical ventilation. She was found to be febrile (102.3 °F) with tachycardia (144 beat per minute). Physical examination was remarkable for decreased breath sounds over the left anterior hemi-thorax and chest X-ray was consistent with pneumonia. She was diagnosed as having respiratory muscle weakness secondary to myasthenia crisis, precipitated by sepsis. Emergent plasmapheresis, steroids and antimicrobial agents were initiated. Pyridostigmine therapy was not immediately instituted due to increased bronchial secretions, but was started later on as there was no improvement despite five cycles of plasmapheresis.
On the eighth day of her hospitalization, she became hypotensive and developed oliguria with acute renal failure likely secondary to acute tubular necrosis (ATN). On the ninth day, she developed abdominal tenderness for which an abdominal computed tomography (CT) scan done, showed free air in the abdominal cavity. An emergent laparotomy revealed peritonitis with gastric cardia perforation, which was repaired.
The patient also had worsening coagulation panel and liver function tests over the course of hospitalization. The prothrombin time (PT) which was normal at the time of admission, later increased to 18.1 s with a decrease in albumin level to 2.1 g/dL (3.2 - 4.8 g/dL). Her bilirubin, alkaline phosphatase (AKP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were all normal at the time of admission (AST of 19 units/L and ALT of 16 units/L). While the alkaline phosphatase and bilirubin levels remained stable, the AST and ALT levels progressively worsened to AST 352 units/L and ALT 369 units/L on the ninth day of hospitalization. The cause of this fulminant hepatic failure was not clear at this time as the level of transaminitis in this patient was not as high as expected from acute viral hepatitis and the etiology was considered to be multifactorial secondary to ischemic hepatopathy and underlying sepsis.
The very next day, after cessation of plasmapheresis, levels of AST and ALT suddenly peaked to 1,618 and 1,018 units/L, respectively. Patient’s clinical course was complicated by the development of acute respiratory distress syndrome and subsequently on the 15th day of hospitalization, she had a fatal cardiac arrest.
The patient’s cause of fulminant hepatic failure was revealed on autopsy.
| Discussion | ▴Top |
Our patient presented with myasthenia crisis and sepsis secondary to pneumonia. Gastric cardiac perforation, worsening respiratory status, and progressively worsening liver function, which resulted in her death, complicated the clinical course. The acute liver failure in the context of severe sepsis in a severely immunocompromised patient has a vast differential diagnosis including ischemic hepatopathy, septic hepatopathy, acute viral hepatitis, drug induced hepatitis and lupus hepatitis.
Ischemic hepatopathy seemed a likely diagnosis given the initial hypoxia and hypotension secondary to sepsis. The pathophysiology of hepatopathy in this scenario involves hypoxic hepatitis presenting with elevated transaminases. Elevation in transaminases in ischemic hepatopathy is characterized by a rapid rise followed by a rapid resolution, provided underlying hypotension has resolved [7]. Our patient did not improve with concomitant intravenous fluid and vasopressor support, but this fact does not exclude ischemic hepatopathy as the diagnosis.
Septicemia can affect liver in two ways: either it causes hypoxic injury leading to ischemic hepatitis or direct injury from released inflammatory mediators, which leads to cholestasis [8]. Cholestasis associated with sepsis is a direct function of interleukins (IL-1, IL-6) [9]. Management of such hepatopathy primarily constitutes treating the shock and its underlying cause.
Drug-induced hepatitis is another common differential diagnosis considered with such high degree of transaminases elevation. Although any drug can cause hepatotoxicity but the commonly reported offenders in clinical practice are acetaminophen, statins, anti-seizure medications, anti-retrovirals and isoniazid. None of these drugs or any other obvious hepatotoxic drugs were used in our patient to substantiate this etiology. Oral contraceptive is another class of medication commonly considered in differential of drug-induced hepatotoxicity but it leads to cholestasis and not anicteric hepatitis as in our case.
Hepatitis A, B, C, D and E viruses can also cause elevation of liver enzymes. In United States, hepatitis A and B viruses are more common epidemiologically as a cause of acute liver failure. According to a report, hepatitis A accounts for 7% cases of acute liver failure and hepatitis B for 2% cases [10]. Hepatitis A is transmitted through feco-oral route and is generally a self-limited infection. Hepatitis E is another virus, which can cause acute liver failure and spreads through feco-oral route. Hepatitis B is transmitted predominantly through transfusion of blood products and sexual contact with an infected person.
Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) are other hepatotropic viruses which need consideration. They both generally affect immunocompromised individuals as a result of de novo infection or reactivation of the latent infection. Illness commonly starts as mononucleosis syndrome with milder elevation of transaminases [11] unlike our patient where much higher levels of transaminases were recorded.
Another possible diagnosis for this patient with a long history of SLE is lupus hepatitis. The hepatic manifestations of lupus are manifold, with the most common presentations being drug-induced hepatic dysfunction and peri-portal hepatitis [12]. Azathioprine use has been reported to be hepatotoxic in 3-10% cases. Various mechanisms explained include hypersensitivity, cholestasis and sinusoidal endothelial cell injury leading to fibrosis and portal hypertension. Hypersensitivity reaction starts within 2 - 3 weeks of drug exposure, whereas sinusoidal endothelial damage can occur anytime between 3 months and 3 years of treatment [13].
The presentation of lupus hepatitis is usually sub-clinical and rarely presents as acute hepatic failure [14]. It has been suggested in the literature that viral etiology of acute liver failure including CMV, hepatitis A, varicella zoster, herpes simplex virus (HSV) and EBV is of high probability in patients with SLE [12]. It is therefore imperative that such patients be thoroughly examined for any suggestive external mucocutaneous manifestations of aforementioned viral agents.
The diagnosis of herpetic hepatitis was confirmed only post-mortem with microscopic examination of hepatic parenchyma revealing herpetic inclusion bodies. On autopsy, the liver was found to be enlarged and mottled with yellow or white, irregularly and randomly distributed (geographic) zones of coagulative necrosis (Fig. 1). Microscopically, the hepatocytes had typical single or multiple intra-nuclear ground glass inclusions (Fig. 2). The inflammatory response was minimal. There was no evidence of an infectious process leading to gastric perforation. The cause of death was stated to be pneumonia with ARDS, fulminant hepatic failure due to disseminated herpes and gastric perforation with peritonitis.
 Click for large image | Figure 1. Liver on post-mortem examination (weight 1,310 g) showing geographic areas of necrosis with random lobular distribution. |
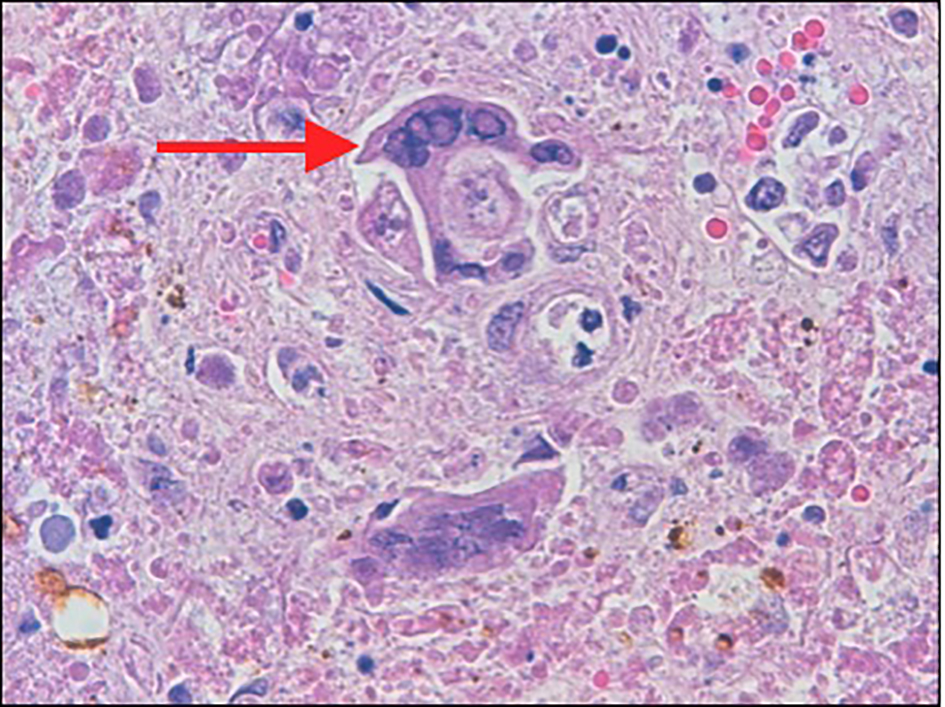 Click for large image | Figure 2. The hepatocytes (arrow) show multinucleation with intra-nuclear viral inclusions at the periphery of necrotic areas. |
Anicteric transaminitis is characteristic of, but not specific to HSV hepatitis [15]. The liver failure is usually evident by transaminitis out of proportion to the bilirubin levels [5]. However, the level of transaminitis in this patient was not as high as expected from acute herpetic hepatitis. A confounding factor in this case was the fact that the patient underwent plasmapheresis, which has been noted in the literature to skew the interpretation of liver function tests in the setting of acute liver failure [16]. Liver function values decrease with plasma exchange and usually return to pretreatment levels within 24 h, which can be interpreted as an artifact of dilution [17]. Indeed in our patient, when plasmapheresis was stopped, there was a sudden spike in both AST and ALT levels (1,618 and 1,018 units/L) on day 10 of hospitalization (Fig. 3).
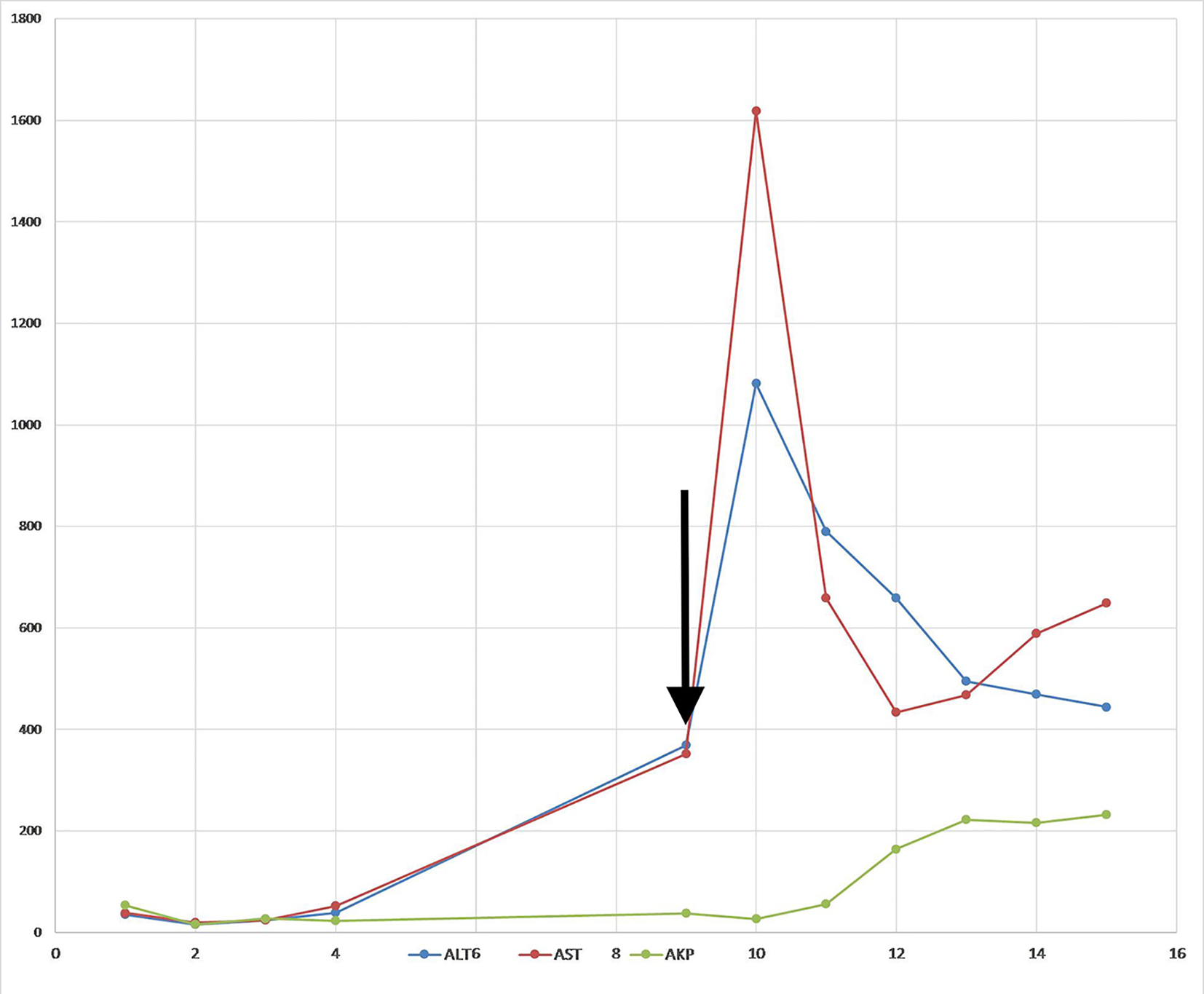 Click for large image | Figure 3. Trend of liver enzymes. Arrow indicates the last day of plasmapheresis. |
HSV hepatitis is one of the rare manifestations of disseminated herpes, as compared to encephalitis and pneumonia [1]. HSV hepatitis, although known to exist in immunocompetent individuals, is predominantly seen in immunocompromised patients and pregnant women. In an analysis of 137 cases done by Norvell et al, 53% patients were immunosuppressed and 23% pregnant [4]. Disseminated infection can be seen in immunocompromised patients with depressed cellular immunity [18]. The dissemination follows either a primary exposure or reactivation of latent infection.
Our patient could have benefited from empiric acyclovir therapy, which as per general consensus, can be instituted without tissue diagnosis [4, 19, 20]. Liver transplantation would also have been a viable option, given the survival rate of over 40% in such cases [1]. The patient then would have been a candidate for lifelong acyclovir prophylaxis [21]. We present this case to remind physicians involved in the care of an immunosuppressed patient with liver failure to be aware of this particularly aggressive, yet treatable form of hepatitis, evident by transaminitis out of proportion to the bilirubin levels. However, in patients undergoing plasmapheresis, liver function values decrease as an artifact of dilution.
Disclosures
None.
Conflicts of Interest
None of the authors have any financial conflicts of interest.
| References | ▴Top |
- Riediger C, Sauer P, Matevossian E, Muller MW, Buchler P, Friess H. Herpes simplex virus sepsis and acute liver failure. Clin Transplant. 2009;23(Suppl 21):37-41.
doi pubmed - Pinna AD, Rakela J, Demetris AJ, Fung JJ. Five cases of fulminant hepatitis due to herpes simplex virus in adults. Dig Dis Sci. 2002;47(4):750-754.
doi pubmed - Kusne S, Schwartz M, Breinig MK, Dummer JS, Lee RE, Selby R, Starzl TE, et al. Herpes simplex virus hepatitis after solid organ transplantation in adults. J Infect Dis. 1991;163(5):1001-1007.
doi pubmed - Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13(10):1428-1434.
doi pubmed - Seksik P, Gozlan J, Guitton C, Galula G, Maury E, Offenstadt G. Fatal herpetic hepatitis in adult following short corticotherapy: a case report. Intensive Care Med. 1999;25(4):415-417.
doi pubmed - Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis. 1997;24(3):334-338.
doi pubmed - Gitlin N, Serio KM. Ischemic hepatitis: widening horizons. Am J Gastroenterol. 1992;87(7):831-836.
pubmed - Aronsohn A, Jensen D. Hepatobiliary manifestations of critically ill and postoperative patients. Clin Liver Dis. 2011;15(1):183-197.
doi pubmed - Gilroy RK, Mailliard ME, Gollan JL. Gastrointestinal disorders of the critically ill. Cholestasis of sepsis. Best Pract Res Clin Gastroenterol. 2003;17(3):357-367.
doi - Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33(1):36-45.
doi pubmed - Talwani R, Gilliam BL, Howell C. Infectious diseases and the liver. Clin Liver Dis. 2011;15(1):111-130.
doi pubmed - Ramos-Casals M, Cuadrado MJ, Alba P, Sanna G, Brito-Zeron P, Bertolaccini L, Babini A, et al. Acute viral infections in patients with systemic lupus erythematosus: description of 23 cases and review of the literature. Medicine (Baltimore). 2008;87(6):311-318.
doi pubmed - Romagnuolo J, Sadowski DC, Lalor E, Jewell L, Thomson AB. Cholestatic hepatocellular injury with azathioprine: a case report and review of the mechanisms of hepatotoxicity. Can J Gastroenterol. 1998;12(7):479-483.
doi pubmed - Chowdhary VR, Crowson CS, Poterucha JJ, Moder KG. Liver involvement in systemic lupus erythematosus: case review of 40 patients. J Rheumatol. 2008;35(11):2159-2164.
doi pubmed - Peters DJ, Greene WH, Ruggiero F, McGarrity TJ. Herpes simplex-induced fulminant hepatitis in adults: a call for empiric therapy. Dig Dis Sci. 2000;45(12):2399-2404.
doi pubmed - Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, Nour B, et al. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006;21(2):96-99.
doi pubmed - Singer AL, Olthoff KM, Kim H, Rand E, Zamir G, Shaked A. Role of plasmapheresis in the management of acute hepatic failure in children. Ann Surg. 2001;234(3):418-424.
doi pubmed - Dinotta F, De Pasquale R, Nasca MR, Tedeschi A, Micali G. Disseminated herpes simplex infection in a HIV+ patient. G Ital Dermatol Venereol. 2009;144(2):205-209.
pubmed - Navaneethan U, Lancaster E, Venkatesh PG, Wang J, Neff GW. Herpes simplex virus hepatitis - it's high time we consider empiric treatment. J Gastrointestin Liver Dis. 2011;20(1):93-96.
pubmed - Glorioso DV, Molloy PJ, Van Thiel DH, Kania RJ. Successful empiric treatment of HSV hepatitis in pregnancy. Case report and review of the literature. Dig Dis Sci. 1996;41(6):1273-1275.
doi pubmed - Montalbano M, Slapak-Green GI, Neff GW. Fulminant hepatic failure from herpes simplex virus: post liver transplantation acyclovir therapy and literature review. Transplant Proc. 2005;37(10):4393-4396.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


