| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 7, July 2018, pages 593-600
This article has been retracted. See J Clin Med Res. 2025 Jun 11;17(6):356.
Malignant Ventricular Arrhythmias Resulting From Drug-Induced QTc Prolongation: A Retrospective Study
Renato De Vecchisa, e, Carmelina Arianob, Giuseppina Di Biasec, Michel Noutsiasd
aPreventive Cardiology and Rehabilitation Unit, DSB 29 “S. Gennaro dei Poveri Hospital”, via S.Gennaro dei Poveri 25, 80136 Napoli, Italy
bDivision of Geriatrics, “Casa Sollievo della Sofferenza” Hospital, viale Cappuccini 2, 71013 San Giovanni Rotondo, Italy
cDivision of Geriatrics, Clinic “S. Maria del Pozzo”, via Pomigliano 40, 80049 Somma Vesuviana, Italy
dMid-German Heart Center, Department of Internal Medicine III, Division of Cardiology, Angiology and Intensive Medical Care, University Hospital Halle, Martin-Luther-University Halle, D-06120 Halle, Germany
eCorresponding Author: Renato De Vecchis, Preventive Cardiology and Rehabilitation Unit, DSB 29 “S. Gennaro dei Poveri Hospital”, via S.Gennaro dei Poveri 25, 80136 Napoli, Italy
Manuscript submitted May 9, 2018, accepted May 15, 2018
Short title: Drug-Induced Long QTc and Arrhythmias
doi: https://doi.org/10.14740/jocmr3470w
| Abstract | ▴Top |
Background: Several drug classes (antiarrhythmics, antimicrobials, antidepressants, phenothiazines, opiates, prokinetics of digestive tract, etc.) have been related to ventricular hyperkinetic arrhythmias such as torsade de pointes (TdP). TdPs are usually heralded by an abnormal prolongation of heart rate-corrected QT interval on the electrocardiogram, so-called drug-induced long heart rate-corrected QT (diLQTc). We don’t know to what extent the drug-induced QTc prolongation is able to predict malignant arrhythmias. Thus we have retrospectively examined the clinical history of patients with diLQTc.
Methods: The case-record, concerning the period from January 2008 to December 2017, was collected from two hospitals. The diLQTc was defined as drug- induced heart rate-corrected QT of ≥ 450 ms or ≥ 470 ms, respectively in male or female patients. The primary purpose was to verify whether in diLQTc patients the length of this electrocardiographic segment was associated with the risk of symptoms or events (TdP, ventricular fibrillation).
Results: A total of 73 validated cases of diLQTc were gathered. Among them, the QTc duration was not able to predict the occurrence of symptoms or events (odds ratio: 0.998; 95% CI: 0.984 to 1.013; P = 0.8821). Likewise, a diQTc lasting longer than 500 ms compared to diQTc comprised between 450 and 500 ms was not associated with an increased risk of arrhythmic events.
Conclusions: In some probably genetically predisposed subjects, the occurrence of symptoms (dizziness, lipothymia, syncope ) and/or documented arrhythmic events (TdP), is related to intake of certain drugs (antiarrhythmics, antimicrobials such as quinolones and macrolides, etc.). Nevertheless, in our diLQTc patients, QTc duration didn’t predict occurrence of symptoms, or arrhythmic events. Thus, other determinants should be postulated to clarify why sometimes diQTc prolongation propitiates ventricular malignant arrhythmias whereas in other cases this arrhythmogenic effect is lacking.
Keywords: Heart rate-corrected QT; Drugs; Ventricular arrhythmias; Prognosis
| Introduction | ▴Top |
The finding of drug-induced long QT (diLQT) on the ECG [1] has a non-negligible impact on the activity of the territorial or hospital cardiologists who are called to examine many surface electrocardiograms of outpatients every week. Sometimes, these electrocardiograms are performed as a routine or preparatory practice for the execution of diagnostic tests (computed tomography and/or magnetic resonance imaging) or surgical interventions, or carried out as a bureaucratic task, i.e., conceived for periodical assessment of job or sport suitability [2], even in individuals who do not have any history or current signs or symptoms of cardiovascular disease. QT is a segment of the QRS complex( Fig. 1) that has been found to be congenitally lengthened in some inherited long QT syndromes (LQTSs) (Jervell and Lange-Nielsen syndrome, Romano-Ward syndrome) [3-4]. However, the present study does not encompass the minute clinical representation and the detailed illustration of the genotypic characterization, instrumental diagnostic methods and of therapeutic measures adopted to counter and/or treat the inherited forms of LQTS.
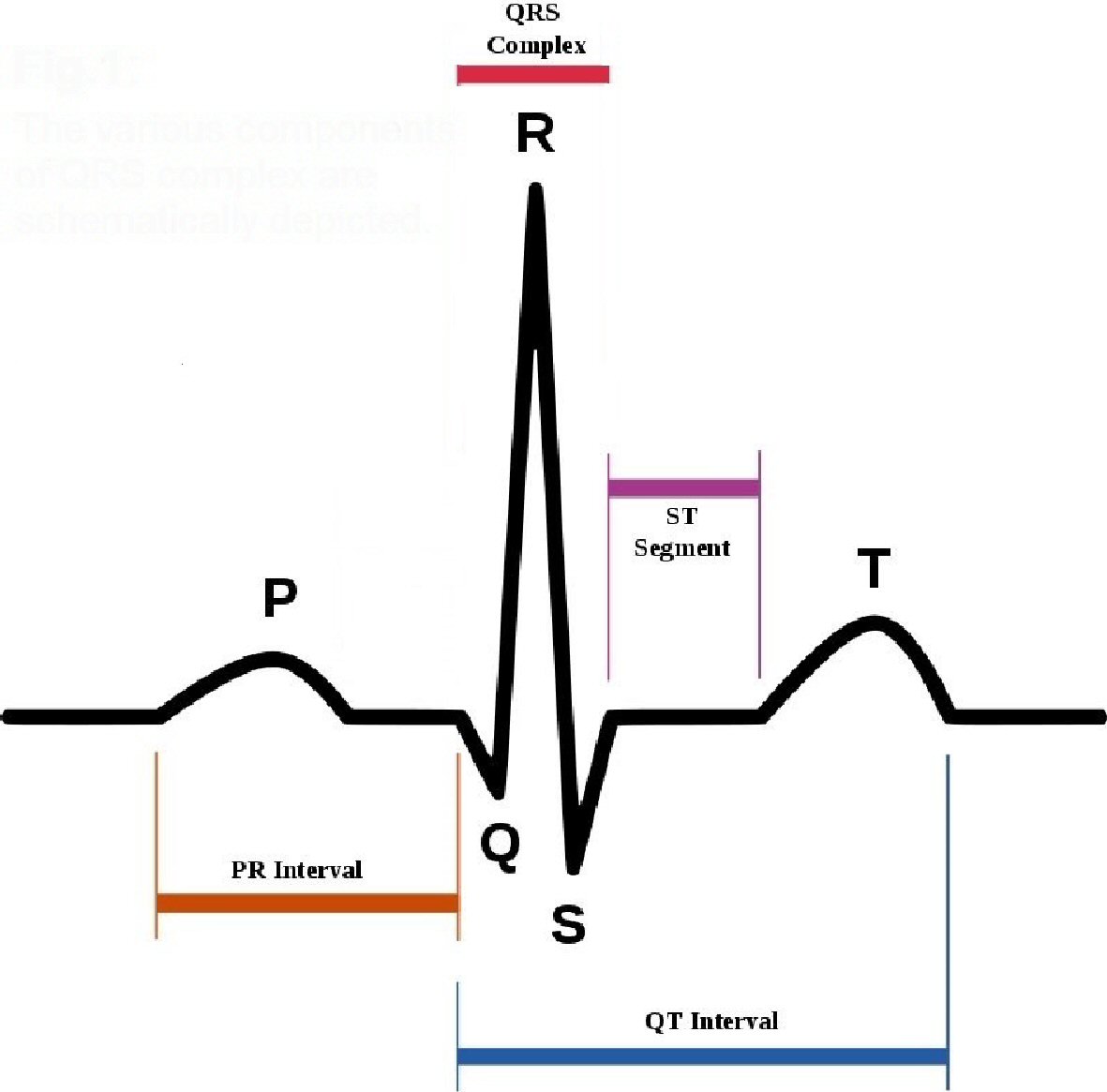 Click for large image | Figure 1. The various components of QRS complex are schematically depicted. |
Conversely, in the present article, our interest focuses rather on the representation of the acquired iatrogenic forms of LQTS, in particular the drug-induced ones, that are related to the use of drugs capable of causing an elongation of this segment of the electrocardiographic trace [5]. Indeed, several drugs involved in the so-called diLQTS have recognized capacities to act on arrhythmogenesis (in particular, a marked antiarrhythmic effect is held by drugs of class I and III of the Vaughan Williams classification) [6-7]. However, in genetically predisposed subjects, also drugs primarily used for non-cardiac diseases, in particular neuroleptic drugs [8], antidepressants [9], antimicrobials [10] and prokinetic agents for high gastrointestinal tract’s motility disorders [11] have been shown to be able to elicit diLQTS. In general, it is reasonable to affirm that QT prolongation is constantly connected to disturbances of ionic homeostasis inside the myocytes [5], that imply their exposure to increased risk of hyperkinetic arrhythmic desynchronization phenomena that may result in polymorphic, i.e. with variable orientation of the electric axis, ventricular tachycardia, so-called “torsade de pointes (TdP)”. This arrhythmia is cause of syncope if of short duration, or it tends to progress to potentially lethal ventricular fibrillation, if rapidly relapsing, or having prolonged duration (≥ 30 s).
The first important point to underline is the need to become familiar with the measurement of QT, in the routine cardiologic diagnostic work. The QT interval or electrical systole includes the entire process of depolarization and ventricular repolarization (Fig. 1). It varies with heart rate by shortening as heart rate increases. For its calculation we accept the formula: QT = 0.39 √C ± 0.04 s, where C is the duration of the cardiac cycle in seconds. In practice, for a heart rate between 100 and 60 beats per minute (bpm), a QT of 0.30 ± 0.04 s is regarded as normal at a frequency of 100 bpm, and for any heart rate value of at least five beats lower, an increase of 0.01 s in QT duration is believed to be normal (e.g., at 95 bpm 0.31 ± 0.04 s, at 80 bpm 0.34 ± 0.04 s, at 60 bpm 0.38 ± 0.04 s). With this calculation we obtain different QT values according to the heart rate. Therefore it’s usually stated that the higher the heart rate, the lower the QT interval will be [12]. As the evaluation of the normal QT duration requires a correction for the heart rate, the calculation of the corrected QT with the use of specific formulas seems of greater value. Among these, the one still most commonly used today, to calculate the so-called “QT interval corrected for heart rate” (QTc), is the Bazett formula: QTc = QT/√RR, where QTc is the QT interval corrected for the heart rate and RR is the duration of the heart cycle interval measured from the beginning of a QRS complex to the following, expressed in seconds (the QT interval is instead expressed in milliseconds) [13]. The normal values of the QTc interval are between 0.30 and 0.44 s (0.45 s for women).
| Methods | ▴Top |
In our study, a certain number of diLQTS/TdP, reported to the pharmacovigilance authority by two centers (“Casa Sollievo della Sofferenza” Hospital, S. Giovanni Rotondo, and S. Maria del Pozzo Clinic, Somma Vesuviana, Italy) were gathered during the 2008 - 2017 decade. Therefore the study has the features of an observational case-control study, undertaken to understand the possible association of use of several drugs with the occurrence of the diLQTS/TdP. In the context of the reports of adverse drug reactions (ADRs) collected during a decade in the two centers, the type of investigation conducted in our study remained limited to the identification of the cases of toxicity with electrocardiographic picture of diLQTS/TdP, while other kinds of manifestations of cardiac toxicity from drugs were not considered. In particular, cases in which the clinical symptomatology was not joined with the electrocardiographic feature of long QT were excluded from the study.
For retrospective enrollment in the study, patients included had to have undergone at least one hospitalization and had to have developed a prolongation of the QT segment that met the following requirements: evidence of a heart rate-corrected QT according to the Bazett formula of ≥ 450 ms if male or of ≥ 470 ms if of female sex. Conversely, patients could or could not have symptoms suggestive of TdP (symptomatic hypotension, dizziness, near-syncope, syncope, shock, cardiac arrest). However they had to be consistently free of right or left bundle branch block (defined according to the current electrocardiographic criteria).
Moreover, the patients implanted with cardiovascular devices were not admitted, because these devices in themselves make the QT measurement impracticable (as in the case of the pacemakers, in which the left ventricular activation delay jeopardizes the validity of QTc measurement due to electrostimulated tracing).
For all drugs involved, an extensive literature search from published reports was performed, which aimed to retrieve all available information regarding the effect of the individual drugs on the QT and/or QTc intervals.
Considering the relative rigidity of the selection criteria, the low prevalence of diLQT in the population and the fact that enrollment involved only two centers, it was estimated that no less than 5 years would be necessary for the collection of an adequate number of cases. However, to increase the case-record and also thanks to the unconditional support of the respective Hospital Directorates, our retrospective research was extended, with the incorporation of the relevant data, so as to include also the 5-year period (2008 - 2012). Finally, the time span of the research period was 10 years: from January, 2008 to December 31, 2017.
Another point to note was the careful compliance with the definition of diLQT.
In fact, for any patient to be enrolled in the retrospective research it was necessary not only that the aforementioned criteria for long QT were satisfied, i.e., a heart rate-corrected QT of ≥ 450 ms if male or of ≥ 470 ms if of female sex, but that a well-defined relationship between abnormal duration of QT and drug intake was also evident. Therefore, each patient proposed for inclusion in the study had to have a documented history of sufficiently prolonged intake of a drug known to have the potential for causing a QT lenghtening. As QT prolongation is subject to critical interference by the potassium, magnesium and calcium serum concentrations [5], researches aimed at identifying the values of these electrolytes were conducted whenever possible. The cause-effect relationship was identified a posteriori due to the study retrospective nature. Moreover, in order to admit a given medication into the list of the causative drugs implicated in the generation of documented cases of diLQT, the following criteria, including the definitions of “certain” or “probable” drug reaction, were adopted.
A drug reaction was assessed as “certain”, when the temporal relationship to the intake of a given drug was plausible, no other cause potentially responsible for the symptom or event was noticeable, disappearance of the LQT/TdP was achievable with the withdrawal of the drug (“positive dechallenge”) whereas the reappearance of the same QT abnormality with or without associated TdP was achievable on re-exposure to the same drug (“positive rechallenge”). Instead, the cause-effect relationship was regarded as “probable” when the temporal relationship with a given drug was plausible, there was no other concomitant factor potentially involved in the generation of LQT/TdP, and a positive dechallenge was found on drug withdrawal. Only cases with “certain” or “probable” drug causality assessment were admitted in our list of patients with ascertained diLQT.
To minimize the risk of false positives, it was agreed that intake of a given drug, as deduced from the history and clinical diary of candidate patients, should have lasted 1 week at least.
Statistical analysis
Patient data, including baseline demographics, comorbidities, hematochemical and echocardiographic data were collected. Data were usually expressed as mean ± SD, whereas median plus interquartile range was used for describing continuous variables having asymmetric distribution of the values around the mean.
In the study design, a logistic regression analysis was also scheduled, conceived to use as outcome variable the composite endpoint “symptoms and clinical events” and to use as exposure variable both QTc duration expressed in ms (continuous variable)and a QTc duration of > 500 ms (dichotomous variable).
This logistic regression analysis was aimed at verifying whether QTc duration can act as reliable predictor of symptoms and/or events (dizziness, near-syncope, syncope, episodes of TdP that are noticeable on the ECG, fatal or non-fatal cardiac arrest). Logistic regression was used also in order to ascertain whether a QTc interval exceeding the arbitrarily established cut-off value of 500 ms was associated with increased risk of symptoms or clinical events.
| Results | ▴Top |
Among the various categories of drugs considered (analgesics, antiarrhythmic drugs, antidepressants, antiepileptics, antimicrobials, cytostatics, gastrointestinal prokinetic agents, neuroleptics etc.) (Fig. 2), we tried to highlight the respective probability of generating asymptomatic increase in duration of QT as opposed to the probability of long QT associated with symptoms (dizziness, near-syncope, syncope, shock, pulseless electrical activity, ventricular fibrillation).
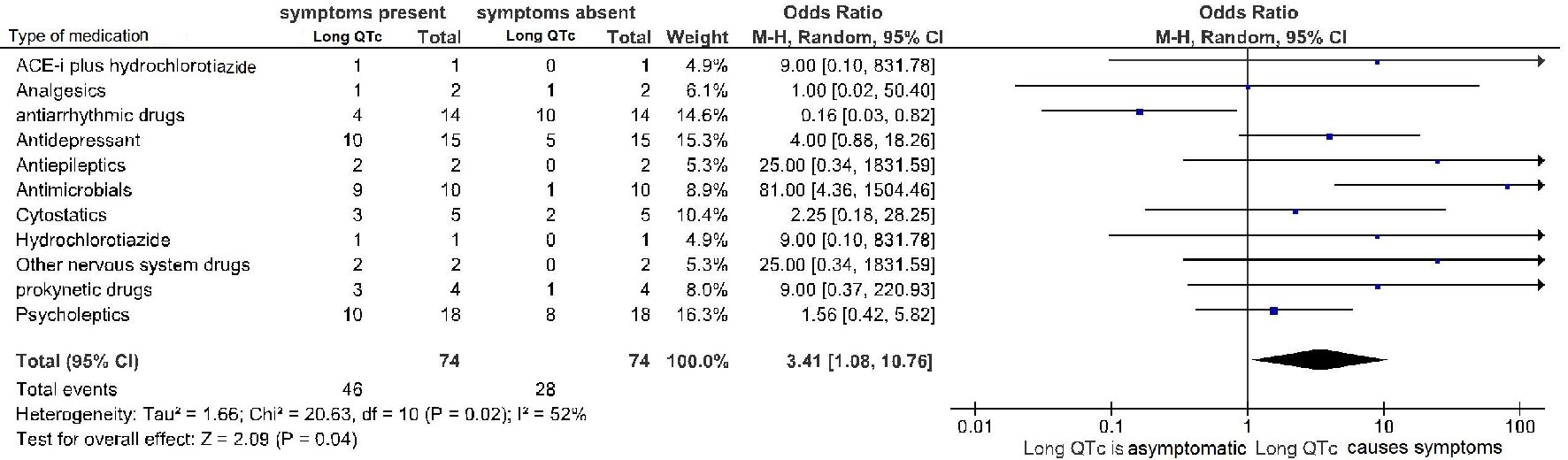 Click for large image | Figure 2. Drug-induced long QTc is a significant predictor of symptoms and arrhythmic events (TdP and ventricular fibrillation). diLQT: drug-induced long QT; QTc: heart rate-corrected QT interval. |
At first, 97 cases were collected, of which 24 could not be confirmed in the validation process (see the flow diagram shown in Figure 3). The reasons were: the re-measured QTc was normal (n = 6), the existence of new-diagnosed right bundle branch block (n = 5) (absent or erroneously undiagnosed in the first evaluation), the existence of a critical electrolyte trouble (n = 5) (absent or erroneously undiagnosed in the first evaluation), the uncertain relation between the drug and the QT prolongation (missed retrieval of normal QT duration after drug withdrawal) (n = 8). Thus, only 73 cases of diLQT were validated in our study and were subsequently entered into the statistical analysis. Patients’ basic information and characteristics are represented in Table 1.
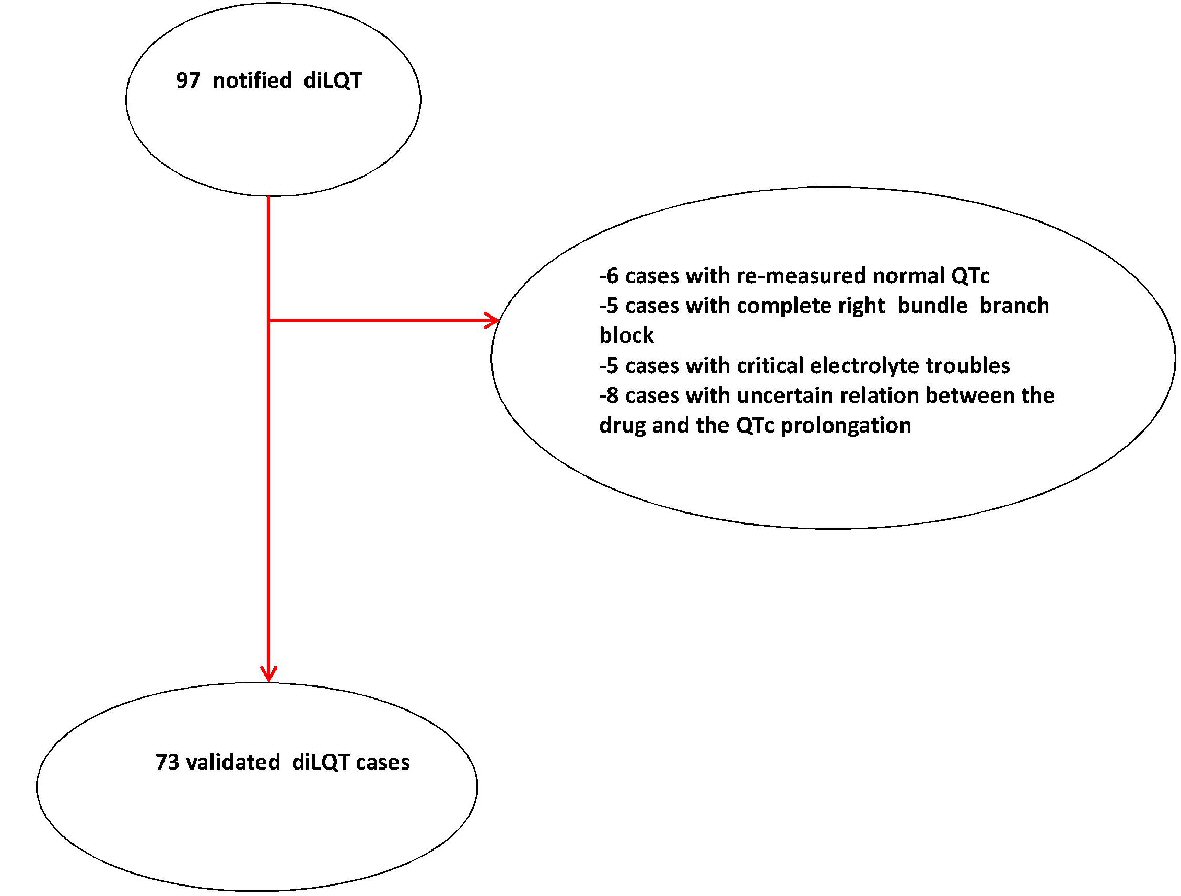 Click for large image | Figure 3. Flowchart of diLQT case validation. diLQT: drug-induced long QT; QTc: QT corrected for heart rate. |
 Click to view | Table1. Patients’ Basic Information and Characteristics |
Electrocardiographic evidence of TdP, that was judged not requiring electric cardioversion was found in 16 patients (22% of the cases), 15 patients (20.5%) underwent cardiac reanimation due to cardiac arrest (ventricular fibrillation or sustained episode of hemodynamically unstable TdP), whereas 15 patients (20.5%) developed syncope (with missing documentation of possible underlying acute electrocardiographic trouble). In 27 patients (37%) diLQTc was not clinically symptomatic nor marked by occurrence of TdP on ECG. On the whole, the cases of long QT accompanied by symptoms or events were 46 (63%) (Table 1 and Figure 4). Median QTc was 501 ms (IQR: 495 - 505). Among the collected cases of diLQTc, 54 (74%) were marked by a QTc duration > 500 ms (Table 1 and Figure 5).
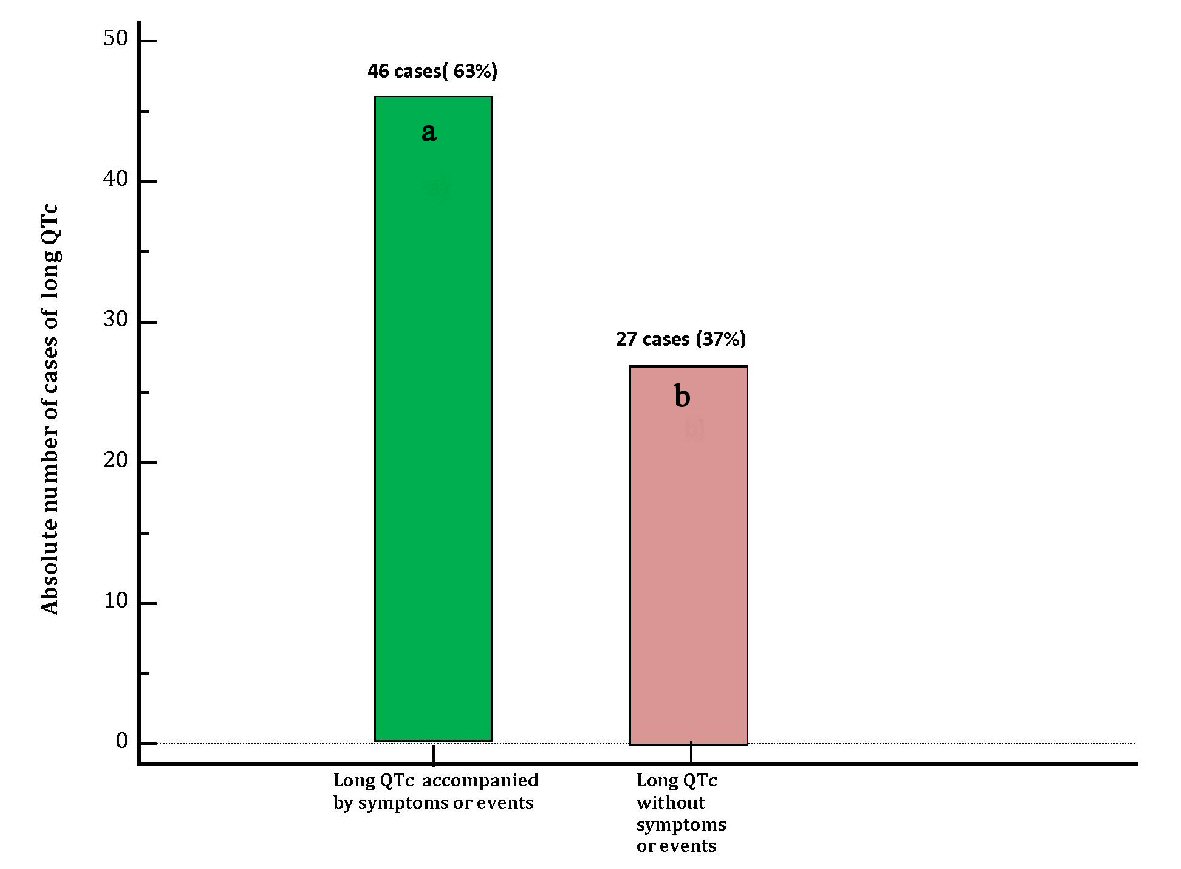 Click for large image | Figure 4. Distribution of cases of diLQT of the present case-record in two groups: (a) Cases of diLQT accompanied by clinical symptoms (dizziness, near-fainting, syncope) or by occurrence of events (TdP on ECG, pulseless electrical activity from prolonged TdP, cardia arrest from ventricular fibrillation) (green bar). (b) Cases of diLQT not associated with clinical symptoms or events, (violet bar). diLQT: drug-induced long QT; QTc: heart rate-corrected QT interval. |
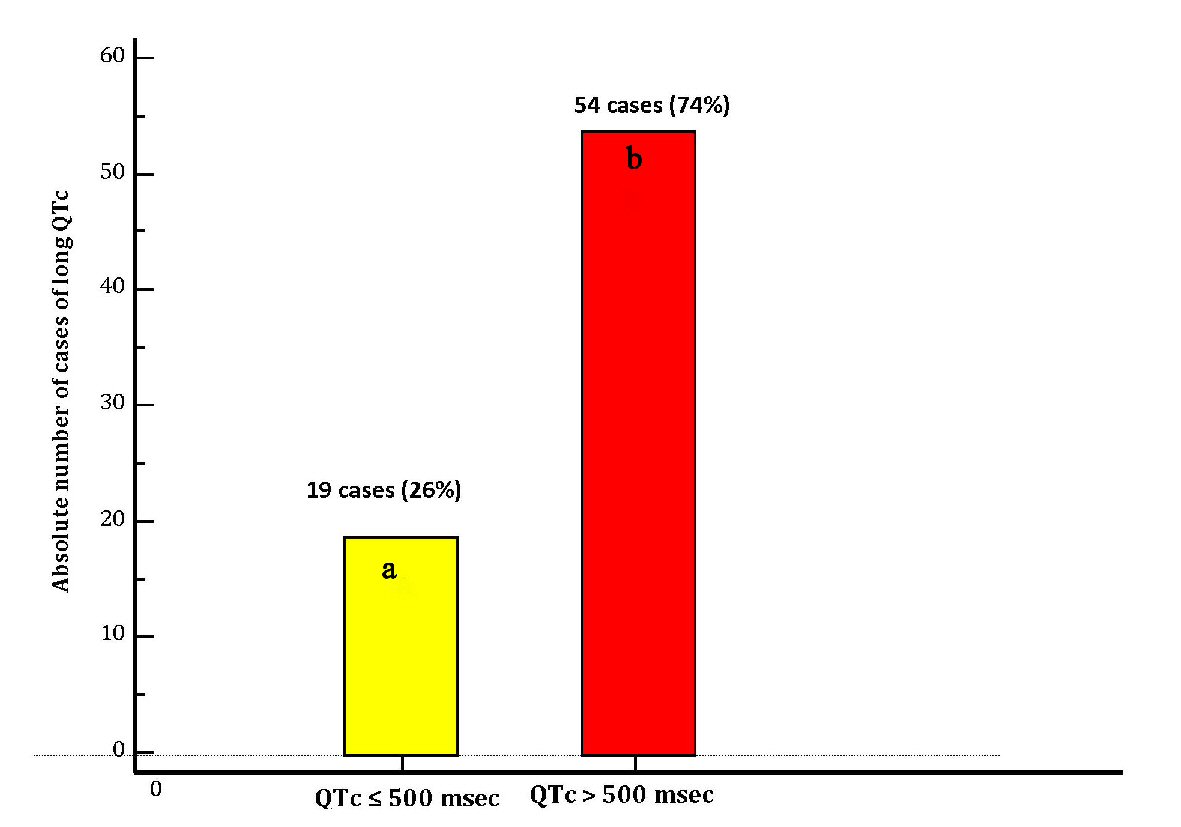 Click for large image | Figure 5. Distribution of cases of diLQT of the present case-record in two groups according to the QTc durations: (a) Cases of diLQT with duration of QTc of ≤ 500 ms (yellow bar). (b) Cases of diLQT marked by a severely increased duration of QTc, i.e. with QTc > 500 ms (red bar). diLQT: drug-induced long QT; QTc: heart rate-corrected QT interval. |
A total of 35 different drugs were assessed as possibly related to LQT, and some of these drugs were assessed as related several times (Table 2). The number of possibly related drugs varied between 1 and 2 per case. Indeed, for 50 cases only one drug was regarded as related, whereas for 23 cases the number of possibly related drugs was 2.
 Click to view | Table 2. All Drugs Involved in Any Iatrogenic Cases of Prolongation of QTc Interval |
In this group of patients with drug-induced LQTc, the duration of this interval on the ECG was not able to predict the occurrence of symptoms or events (odds ratio: 0.998; 95% CI: 0.984 to 1.013; P = 0.8821) (Table 3). Moreover, in this series of patients, a QTc lasting longer than 500 ms compared to QTc comprised between 450 and 500 ms was not associated with an increased risk of cardiac arrest or symptoms (dizziness, near-syncope, syncope) ( odds ratio: 0.533; 95% CI: 0.162 to 1.752; P = 0.3006) (Table 3).
 Click to view | Table 3. Logistic Regression Analysis Including Patients With diLQTc |
Nevertheless, among these patients, all with QTc interval of ≥ 450 ms, even though QTc duration proved to be unable to reliably discriminate asymptomatic cases of diLQTc from those marked by clinical symptoms or events, altogether patients with symptoms or events were significantly more numerous compared to those with asymptomatic LQTc (odds ratio 3.41, 95% CI: 1.08 to 10.76, p = 0.04) (Fig. 2).
| Discussion | ▴Top |
In our study 73 validated cases of diLQTc were identified. A total of 35 different drugs were involved in these cases. QTc duration was unable to reliably discriminate asymptomatic cases from those with clinical symptoms or events. Moreover, in this series the number of patients with symptoms or events was significantly higher than that of patients without symptoms.
A first concept that deserves attention is the improved awareness of the mechanisms involved in the drug-related arrhythmogenesis. In fact, the concept that the “antiarrhythmic” term is improper has recently gained ground, since the pharmacologic agents included in the Vaughan Williams classification of the antiarrhythmic drugs are really provided with ambivalent activity, both pro-arrhythmic and antiarrhythmic [14]. In this regard, the only exception is the Vaughan Williams class II, whose drugs are essentially represented by beta-blockers, which increase myocardial electrical stability and therefore consistently prevent or attenuate hyperkinetic ventricular arrhythmias.
This increased awareness of the paradoxical arrhythmogenic potential of some “antiarrhythmic” drugs has led to a significant change in the prescriptive behavior of doctors especially as regards the drugs of the class IA of Vaughan Williams. In fact, today these drugs either are no longer on the market (quinidine polygalacturonate, moricizine, ajmaline) or are prescribed for relatively short periods of time (hydroquinidine retard, procainamide); thus an important source of cases of diLQTc has been suppressed.
Another aspect is the finding of the apparent clinical harmlessness of long QTc related to amiodarone. In our series the antiarrhythmic drugs identified as probable cause of QTc prolongation were three: amiodarone (10 ascertained cases, of which only three with symptoms), dronedarone (two ascertained cases, both asymptomatic); flecainide (two ascertained cases, in which one with symptoms). It is essential to underline here that the category of antiarrhythmic drugs involved in a QT prolongation was primarily represented by amiodarone (10 cases on a total of 14 cases of antiarrhythmic-induced QTc prolongation). So it would perhaps be appropriate to assert that amiodarone-related long QTc is associated with a low risk of symptomatology (syncope, shock, sudden death), while it is not at all certain that this same reassuring confirmation holds true for other “antiarrhythmic drugs” .
Furthermore, for long QT caused by other categories of drugs (analgesics, antidepressants, antiepileptics, cytostatics, gastrointestinal prokinetic drugs, neuroleptics), a marked potential risk for clinical manifestations has not been documented. Conversely, in the case of long QTc related to antimicrobials, an increased risk of symptoms (syncope) or events (arrhythmic death) would be evident (Fig. 2).
From the thorough analysis of our case-record, some further useful insights for the Discussion ensue. Firstly, there are no well-coded causal relationship patterns in the clinical presentation of the drug-related long QTc. This means that in different subjects a new-onset long QTc may arise after a few days of exposure to a given drug [6], or alternatively, as a gradual process within a prolonged period ( months or years) of pharmacologic exposure [6]. In this context, a genetically predetermined susceptibility to the emergence of this anomaly, to a variable extent in the inter-individual comparison, could play a non-negligible role. Furthermore, there may be important differences in the clinical significance of long QTc, considering that, when dependent on amiodarone, this condition, even in the presence of QTc values exceeding 500 ms, is rarely marked by the occurrence of malignant arrhythmias. Conversely, its causal relationship with the quinidine (Vaughan Williams class IA) [6] or its causal association with some antimicrobials such as quinolones and macrolides [10] has been related to a high risk of TdP and cardiac arrest. As regards the studies that have addressed the issue of drug- induced QT lengthening, a particular mention is due to the recent cohort study by Sarganas et al [15]. In this study a detailed report concerning symptomatic drug-induced long QT syndrome (LQTS) has involved 51 collaborating hospitals in a well-defined patient population derived from the city of Berlin (Germany) between 2008 and 2011. A total of 170 patients with possible LQTS/TdP were reported to the Pharmacovigilance Center of whom 58 cases were confirmed in an accurate validation process. The majority (66%) of these cases were female and 60% had developed LQTS/TdP in the outpatient setting. Thirty-five (60%) of 58 confirmed cases were assessed as drug-related based on a standardized causality assessment. Drugs identified as related in more than two cases were metoclopramide, amiodarone, melperone, citalopram, and levomethadone.
Conversely, in our study including a total of 73 patients with iatrogenic QTc prolongation (QTc longer than 450 ms in all cases) the possible difference in the occurrence of symptoms and/or arrhythmic events depending on the drug-induced QTc duration has been investigated.
In our study, no significant relationship has emerged between QTc duration and risk of TdP-related symptoms. However, a recent study would demonstrate that even asymptomatic diLQTS has ominous prognostic consequences [16]. In that study, patients with severe drug-induced QTc prolongation (QTc > 500 ms) had a 4-fold higher mortality rate during follow-up compared with patients with a QTc < 500 ms [16].
In truth, the findings ensuing from our retrospective study partially disclaim the concept that a linear relationship would exist between QTc duration and risk of malignant ventricular arrhythmias. Perhaps such relation might be demonstrated in individuals with a concomitant cardiomyopathy. Instead, the patients enrolled in our retrospective study were relatively young and those affected by chronic heart failure attained only 20.5% of the total (Table 1).
In conclusion, it is reasonable to affirm that further studies, in particular prospective cohort studies, concerning drug-induced long QTc are warranted, especially within the subset of patients with chronic heart failure whose percentage number was too low in our retrospective study. Such a study would be able to establish whether the risk of sudden death is critically conditioned by duration of iatrogenic QTc lengthening among patients with ascertained cardiac dysfunction.
Study limitations
The excessive heterogeneity of the case-record was not useful for the formulation of convincing hypotheses with respect to the primary question of assessing the risk that a diLQTc generates clinically significant symptoms or cardiac events.
Conclusions
In our study about patients with diLQTc, using logistic regression analysis we have found that the risk of significant clinical symptoms (dizziness, syncope as a clinical-hemodynamic correlate of TdP, ventricular fibrillation elicited by TdP) is not predicted by the duration of the drug-induced LQT. Therefore the determinants responsible for triggering of malignant hyperkinetic arrhythmias in patients with diLQTc remain unknown or uncertain. In other words it is not clear whether the risk results from a predisposing genetic substratum and/or it is related to cardiotoxic and arrhythmogenic characteristics of a given drug, which are not accurately mirrored by the extent of QTc increase on ECG. Alternatively a concomitant undiagnosed cardiomyopathy could be hypothesized as a factor able to trigger TdP or ventricular fibrillation as a consequence of a diLQTc that would remain instead asymptomatic in a patient without cardiomyopathy.
Conflict of Interest
Michel Noutsias has received grants by the Deutsche Forschungsgemeinschaft (DFG) through the Sonderforschungsbereich Transregio19 “Inflammatory Cardiomyopathy” (SFBTR19; TP B2), and by the University Hospital Giessen and Marburg Foundation Grant “T cell functionality” (UKGM 10/2009). Michel Noutsias has been consultant to the Institute for Cardiac Diagnosis and Therapy, Berlin (June, 2004 - June, 2008) and has received honoraria for presentations and/or participated in advisory boards from AstraZeneca, Bayer, Boehringer Ingelheim, Fresenius, Miltenyi Biotech, Novartis, Pfizer and Zoll. Renato De Vecchis, Carmelina Ariano and Giuseppina Di Biase do not have any conflict of interest to declare.
| References | ▴Top |
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013-1022.
doi pubmed - Asif IM, Drezner JA. Sudden cardiac death and preparticipation screening: the debate continues-in support of electrocardiogram-inclusive preparticipation screening. Prog Cardiovasc Dis. 2012;54(5):445-450.
doi pubmed - Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54(1):59-68.
doi - Chiang CE. Congenital and acquired long QT syndrome. Current concepts and management. Cardiol Rev. 2004;12(4):222-234.
doi - El-Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin Electrophysiol. 2018;41(4):414-421.
doi pubmed - Roden DM, Woosley RL, Primm RK. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986;111(6):1088-1093.
doi - Yalta K, Turgut O, Yilmaz A, Yilmaz MB, Kendirlioglu O, Karadas F. Torsades de pointes with a severely prolonged QT interval induced by an initial low dose sotalol intake. Int J Cardiol. 2007;116(3):e95-97.
doi pubmed - Palanca-Maresca I, Ruiz-Antoran B, Centeno-Soto GA, Forti-Buratti MA, Siles A, Usano A, Avendano-Sola C. Prevalence and risk factors of prolonged corrected QT interval among children and adolescents treated with antipsychotic medications: a long-term follow-up in a real-world population. J Clin Psychopharmacol. 2017;37(1):78-83.
doi pubmed - Beach SR, Kostis WJ, Celano CM, Januzzi JL, Ruskin JN, Noseworthy PA, Huffman JC. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry. 2014;75(5):e441-449.
doi pubmed - Mason JW. Antimicrobials and QT prolongation. J Antimicrob Chemother. 2017;72(5):1272-1274.
doi pubmed - Benatar A, Feenstra A, Decraene T, Vandenplas Y. Effects of cisapride on corrected QT interval, heart rate, and rhythm in infants undergoing polysomnography. Pediatrics. 2000;106(6):E85.
doi pubmed - Levine E, Rosero SZ, Budzikowski AS, Moss AJ, Zareba W, Daubert JP. Congenital long QT syndrome: considerations for primary care physicians. Cleve Clin J Med. 2008;75(8):591-600.
doi pubmed - Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353-370.
- Camm AJ. Hopes and disappointments with antiarrhythmic drugs. Int J Cardiol. 2017;237:71-74.
doi pubmed - Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16(1):101-108.
doi pubmed - Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(4):315-325.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


