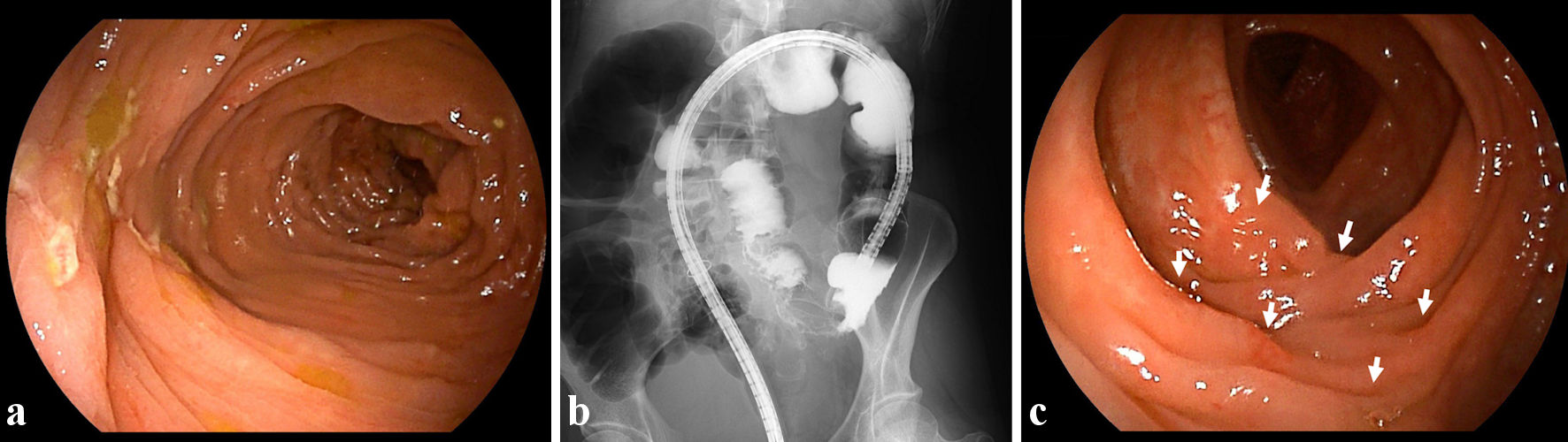
Figure 1. Findings from colonoscopy and radiology. (a) On admission, colonoscopic image shows skipped ulcers fused with the adjacent ulcer and/or longitudinally aligned in the mucosa of the transverse colon. (b) On admission, radiological findings with contrast media reveal longitudinal ulcers as well as edematous changes and stenoses in the transverse colon. (c) At 8 weeks after starting ustekinumab, colonoscopic image shows skipped ulcer scars (white arrows) in the transverse colon.
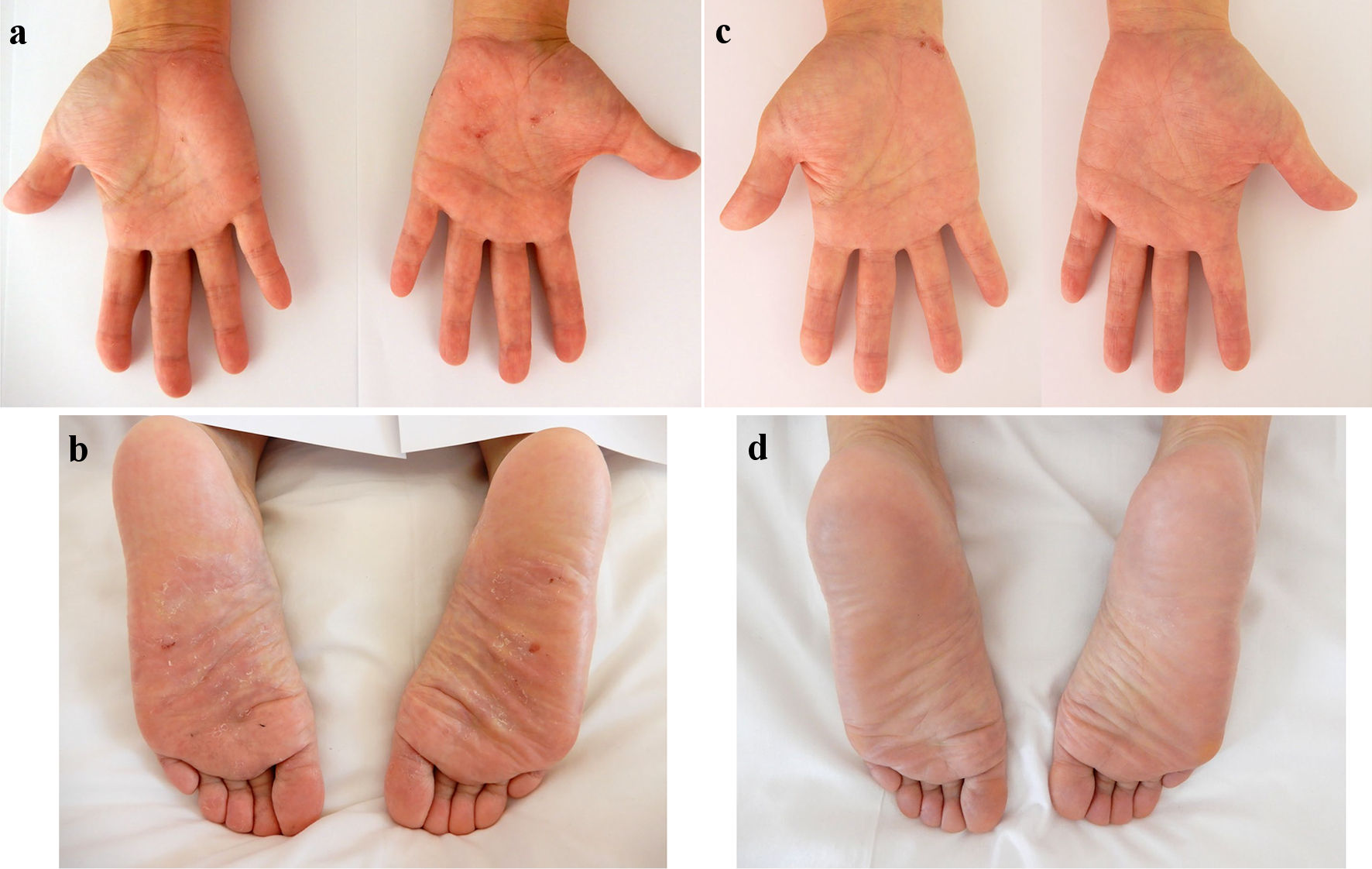
Figure 2. Skin findings of palmoplantar pustulosis on the palms and soles. A small number of blisters and pustules, and scattered scales on diffuse erythematous skin were present on both the palms (a) and soles (b) on admission. At 8 weeks after starting ustekinumab, focal scale is present on the right palm (c) without blisters, pustules, scales, or erythema on the left palm and both soles (c, d).
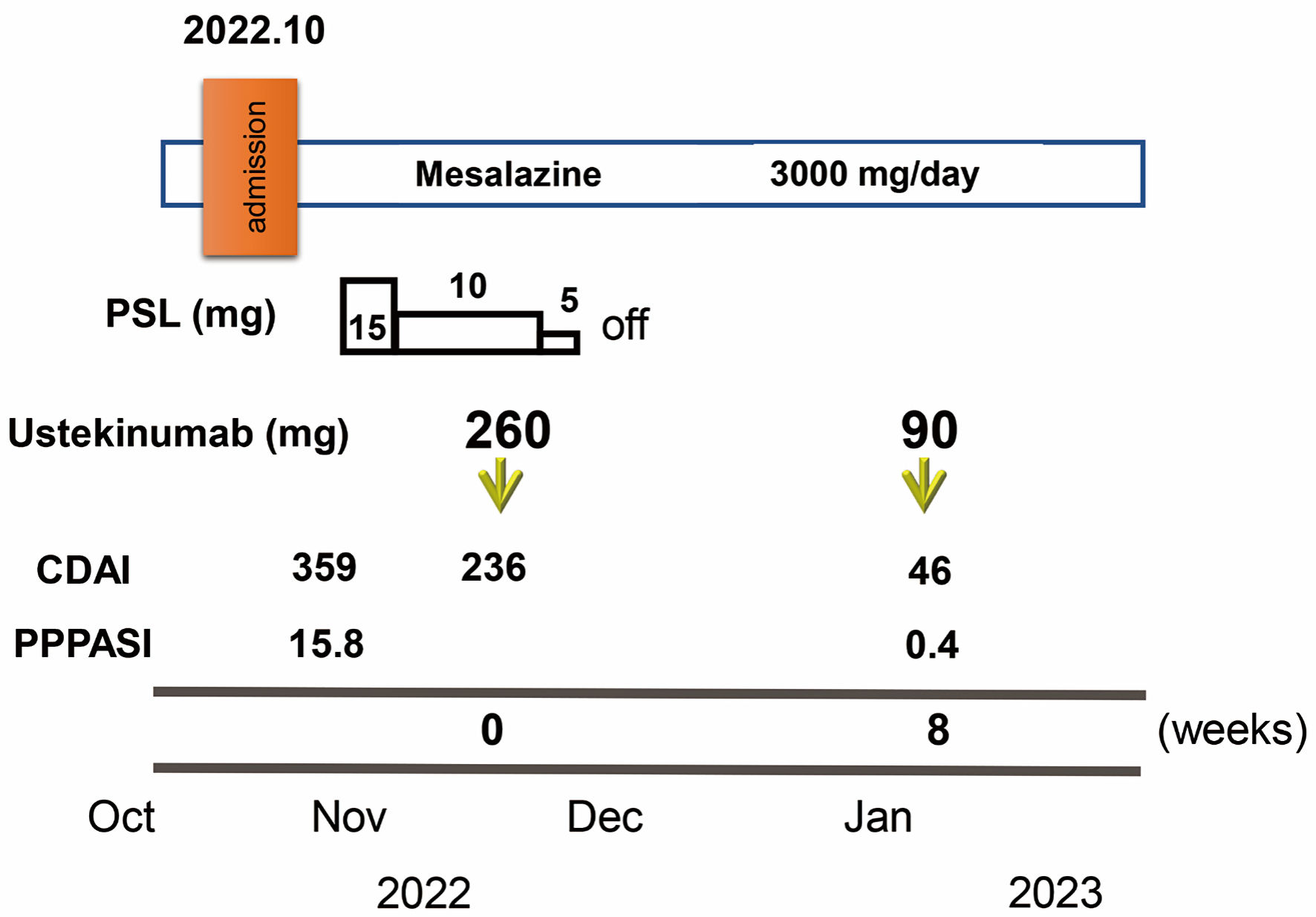
Figure 3. Clinical course through 8 weeks. Crohn’s disease activity index (CDAI) and palmoplantar pustulosis area and severity index (PPPASI) on admission were 359 and 15.8, respectively. Oral prednisolone (PSL) was started at 15 mg/day accompanied by mesalazine at 3,000 mg/day. CDAI decreased to 236. In November 2022, ustekinumab was started at 260 mg based on 6 mg/kg in week 0 with the aim of clinical remission of CD and PPP. The dose of PSL was tapered off in 1 week. At 8 weeks after starting ustekinumab, clinical remission was achieved with a CDAI of 46 and PPP manifestations on the palms and soles were markedly improved (PPPASI: 0.4).


