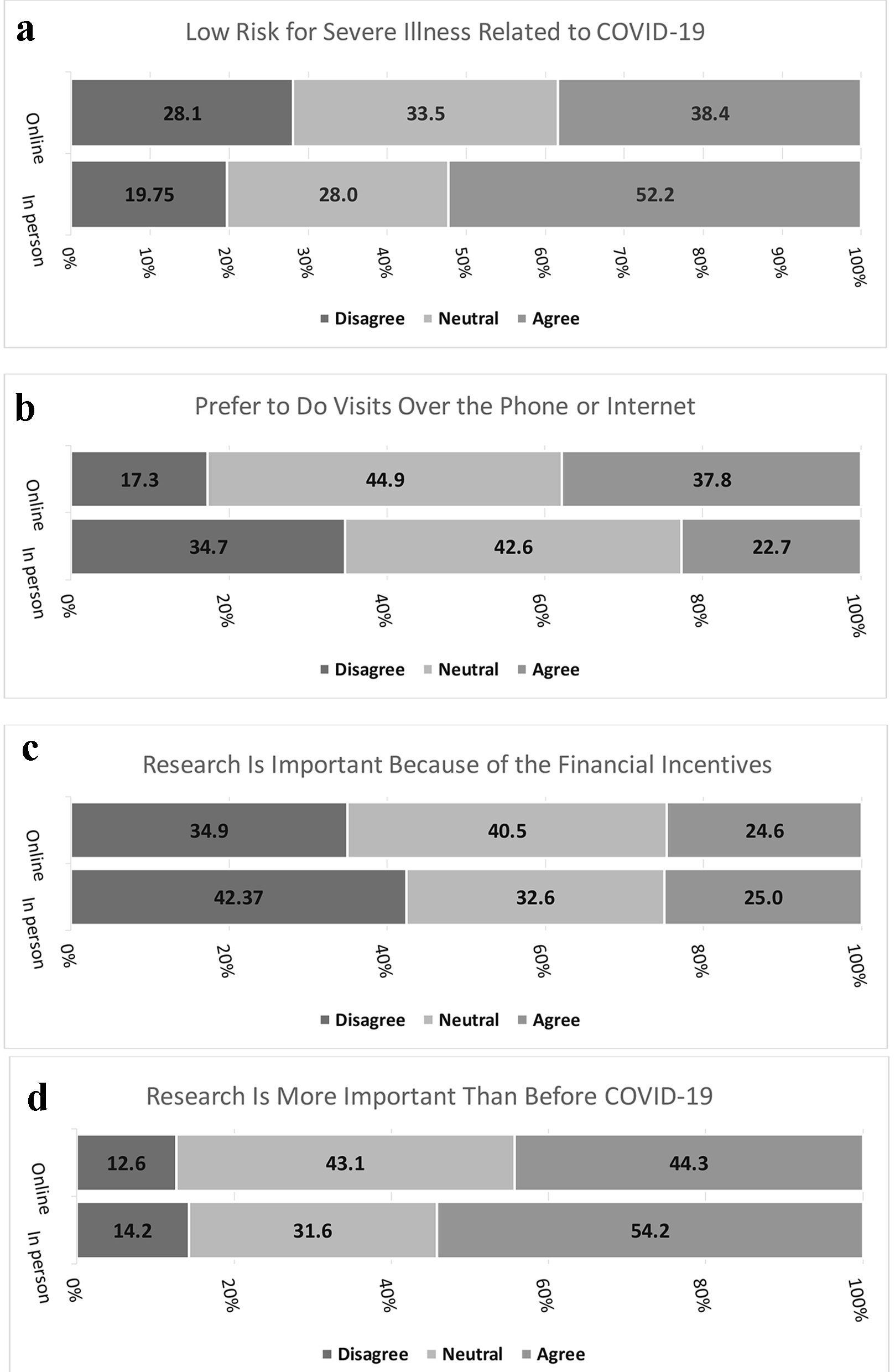
Figure 1. (a) Low risk for severe illness related to COVID-19. (b) Prefer to do visits over the phone or internet. (c) Research is important because of the financial incentives. (d) Research is more important than before COVID-19. COVID-19: coronavirus disease 2019.