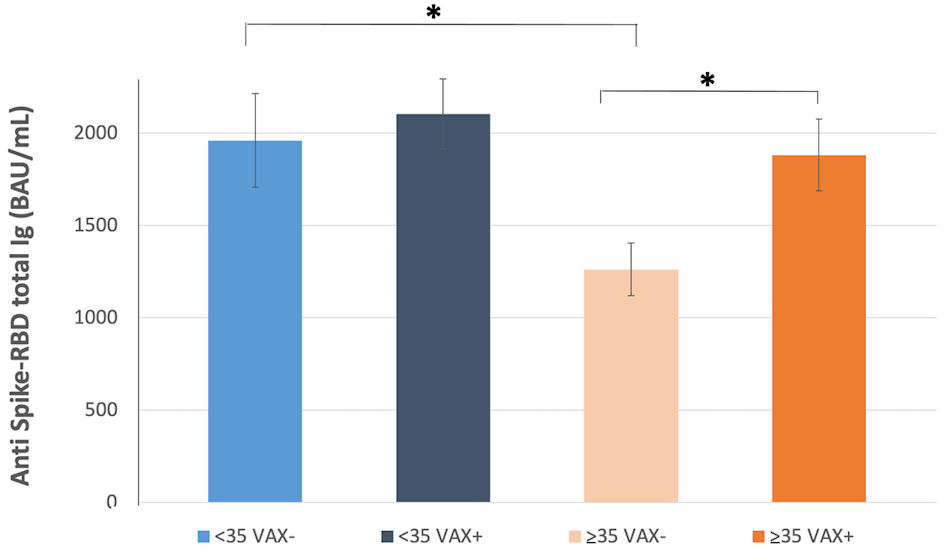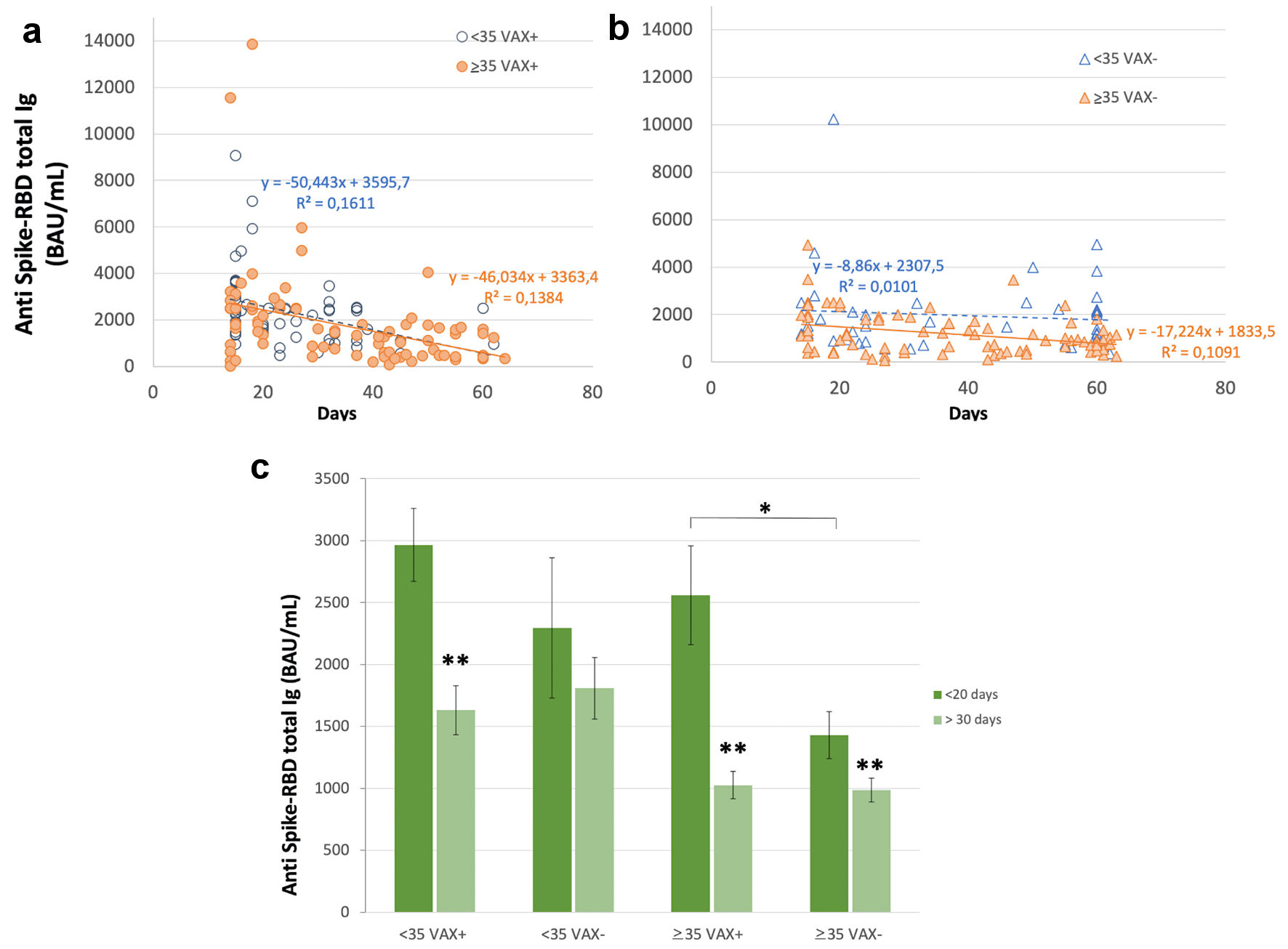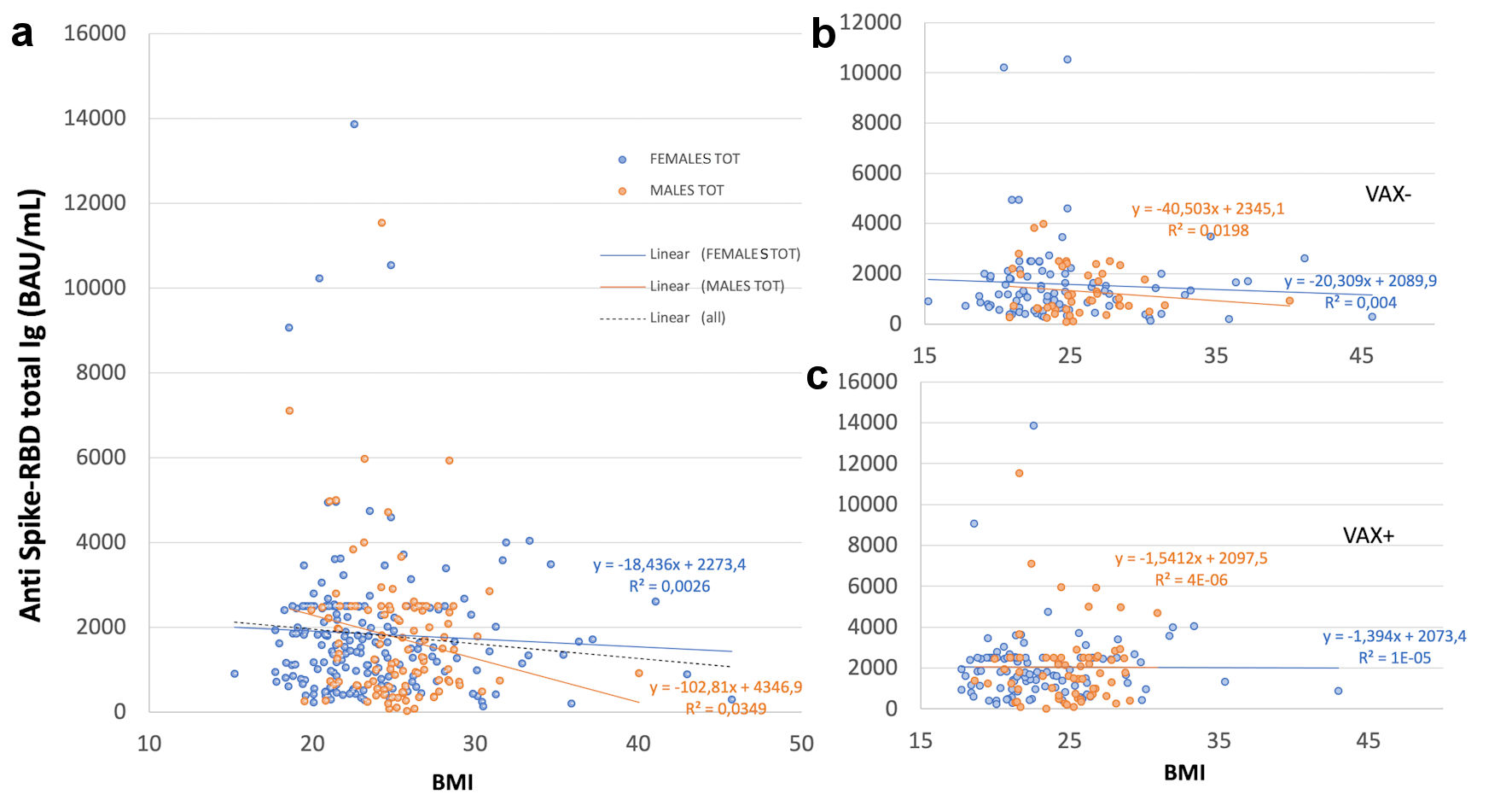
Figure 1. Correlation of total antibodies to the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) and body mass index (BMI) after complete BNT162b2 vaccination in (a), all analyzed HCWs (males and females) vaccinated for seasonal influenza (VAX+) compared to not vaccinated for seasonal influenza (VAX-), the black dotted trend line indicates regression between all analyzed subjects; and in (b), VAX+ and (c), VAX- individuals. Simple regression analysis with P = 0.054 and 0.484 for total males and females, respectively (a); P = 0.356 and 0.560 for VAX- males/females (b); P = 0.148 and 0.973 for VAX+ males/females (c). SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; HCWs: healthcare workers; BAU: binding antibody unit.