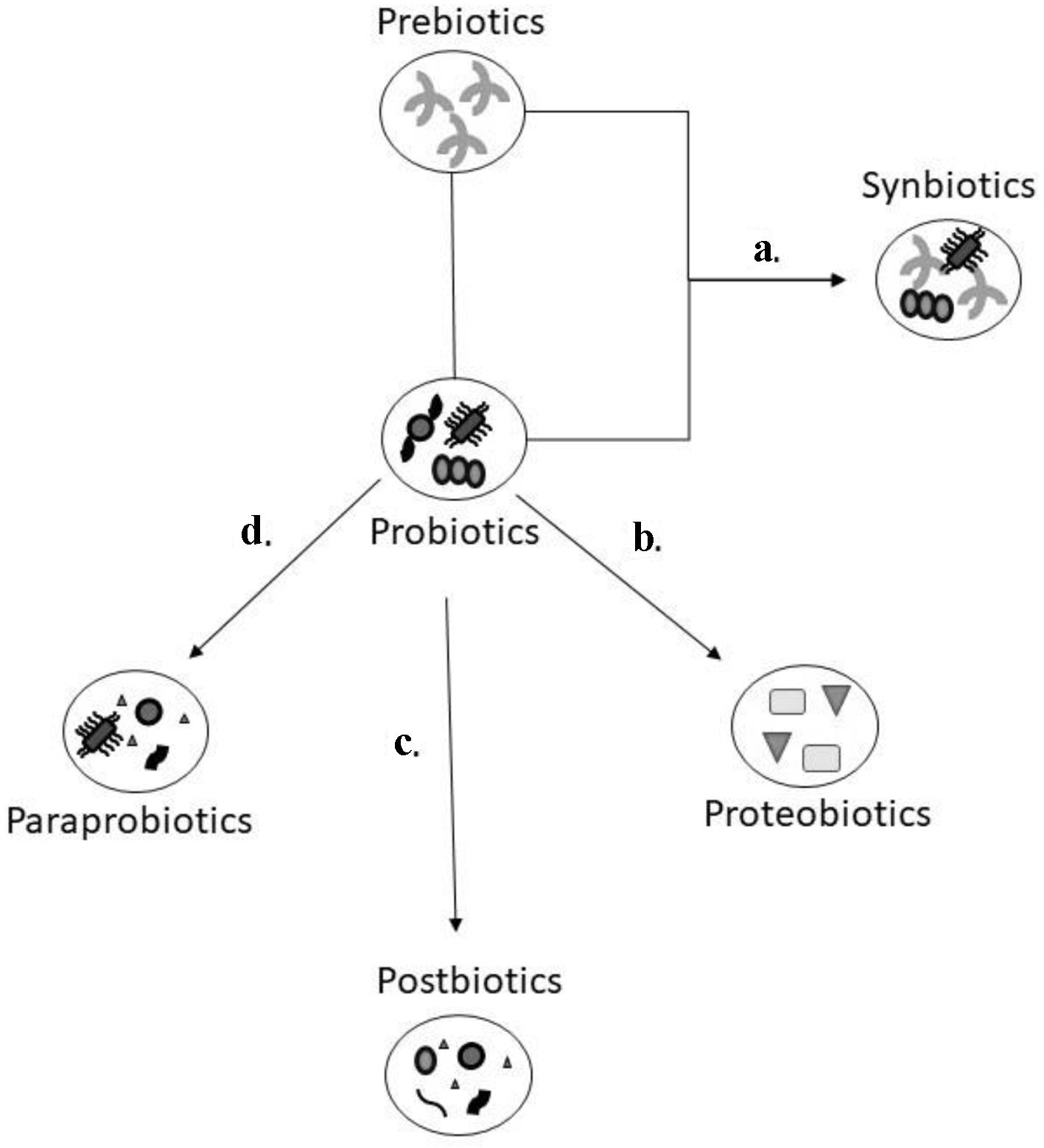
Figure 1. Schematic model of the various gut biotics. Prebiotics are dietary fibre that help with the growth of probiotics which are microorganisms that can provide a health benefit. The combination of prebiotics and probiotics (a) is referred as synbiotics as it is felt there is a synergistic effect due to this combination. Postbiotics, paraprobiotics, and proteobiotics are considered non-viable bacterial products or metabolic by products from probiotic microbes. The natural metabolites through the process of fermentation (b) are isolated from the culture media of probiotics which are identified as proteobiotics. When probiotics are treated through sonication, chemical, or enzymatic processes (c), they are broken down to functional bioactive compounds, referred to as postbiotics. Using radiation, heating, pasteurization and other inactivation methods (d), probiotics are converted to non-viable microbial cells known as paraprobiotics.
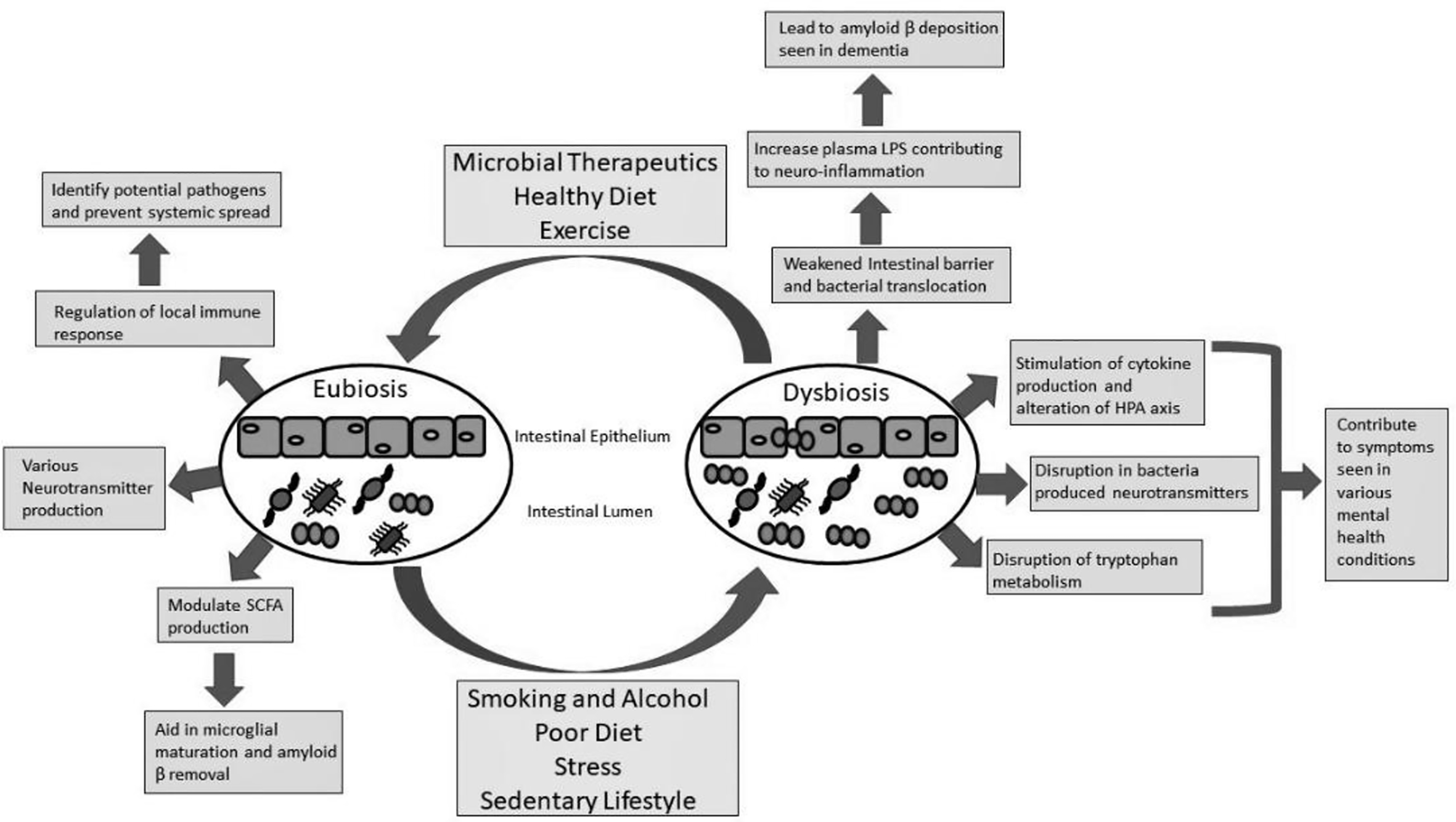
Figure 2. The role of the gut microbiome in neurocognitive and metal health conditions. In a state of eubiosis, the gut bacteria are controlled by the host immune system to prevent systemic spread. Since many of the gut bacteria have a polysaccharide coating the immune system can recognize potential pathogens from the commensal organism. This can prevent systemic spread and inflammation that has been shown to play a role in various mental health conditions. Different species of bacteria in the gut microbiome can produce different neurotransmitters, such as serotonin through tryptophan metabolism. Short-chain fatty acids (SCFAs) are also produced by different gut bacteria and have been found to play a role in microglial maturation which can help remove amyloid β peptide (the major component in Alzheimer’s disease (AD)). Various factors, such as lifestyle and diet, can lead to microbial dysbiosis. A weakened intestinal barrier can result in bacterial translocation causing increased lipopolysaccharide (LPS) serum levels. The result is systemic and neuroinflammation that can contribute to amyloid β deposition leading to AD. The systemic inflammation can become chronic and lead to an alteration of the hypothalamic pituitary adrenal axis (HPA) and has been linked to various mental health conditions. The disrupted microbiome can affect neurotransmitter production and tryptophan metabolism, again impacting the host’s mental health.

