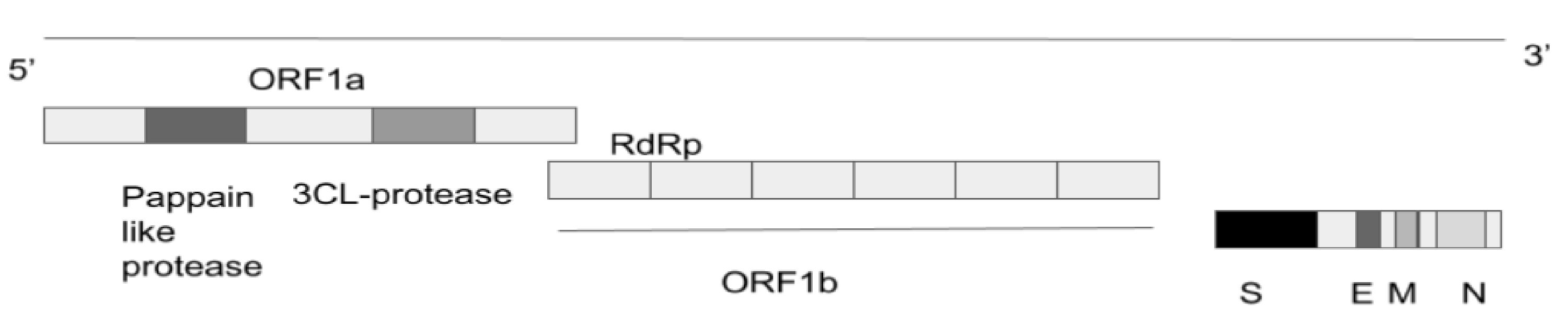
Figure 1. SARS-CoV-2 genome. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; N: nucleocapsid; M: membrane; S: spike; E: envelope; RdRp: RNA-dependent RNA polymerase; ORF: open reading frame.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 13, Number 6, June 2021, pages 317-325
Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know?
Figure

Tables
| Mutation | Mutation location | Role of the mutation | Variant |
|---|---|---|---|
| RDB: receptor binding domain; ACE2: angiotensin-converting enzyme 2; ORF: open reading frame. | |||
| S:N501 | RDB | May increase ACE2 binding. Tests in Pfizer-BioNTech and Moderna vaccinated individuals suggest reduction in neutralisation. | UK (20I/501Y.V1) |
| South Africa (20H/501Y.V2) | |||
| Brazil (20J/501Y.V3) | |||
| S:E484 | RDB | May increase ACE2 binding. | South Africa (20H/501Y.V2) |
| Brazil (20J/501Y.V3) | |||
| Brazil (20B/S.484K) | |||
| S:H69- | Spike N-terminal domain | May alter recognition by antibodies. | UK (20I/501Y.V1)/B.1.1.7 |
| S:Q677 | Near to both outside the furin building pocket; important for S1/S2 cleavage | Hypothetically thought to influence the S1/S2 cleavage. | 20G (20C-US clade) |
| S:Y453F | RDB | May increase ACE2 binding. | Cluster 5 “mink” variant seen in minks in the Netherlands |
| Confer resistance to the antibody in the Regeneron cocktail. | |||
| S:S477 | RDB | Slight increase in ACE2 binding. | |
| Confer resistance to antibody and some convalescent sera and a modest increase in infectivity. | |||
| S:L18F | Spike N-terminal domain | Reduction in binding for monoclonal antibody. | South Africa (20H/501Y.V2) |
| Brazil (20J/501Y.V3) | |||
| S:Y144- | Spike N-terminal domain | Associated with antibody escape. | UK (20I/501Y.V1), 20A/S:484K |
| S:H655 | Brazil (20J/501Y.V3) | ||
| S:P681 | Near the furin cleavage site | It may reduce antibody recognition. | UK (20I/501Y.V1) |
| S:K417 | RDB | It may escape antibody binding and decrease binding to ACE2 receptor. | South Africa (20H/501Y.V2) |
| Brazil (20J/501Y.V3) | |||
| ORF1a:S3675 | It is a three amino acids deletion in ORF1a at positions 3675-5677. | 20C/S:484K and 20A/S:484K | |
| Signs and symptoms | Original variant | Variant of interest | Variant of concern | Variant of high consequence |
|---|---|---|---|---|
| WHO: World Health Organisation; CDC: Center of Disease Control and Prevention; EUA: Emergency Use Authorization. | ||||
| Symptoms | ||||
| Cough | 28% | 35% | ||
| Fatigue/weakness | 29% | 32% | ||
| Headache | 30% | 32% | ||
| Muscle aches | 21% | 25% | ||
| Sore throat | 19% | 22% | ||
| Fever | 20% | 22% | ||
| Loss of taste | 19% | 16% | ||
| Loss of smell | 19% | 15% | ||
| Disease transmissibility | Yes | No | Yes | Yes |
| Disease severity | Less | Less | More | More |
| Diagnostic testing failures | No | No | No | Yes |
| Treatment | Potential reduction in neutralization by monoclonal antibody treatments | B.1.1.7 variants have minimal, while P.1, B.1.351 variants have moderate and B.1.427, B.1.429 have significant impact on EUA therapeutics and monoclonal antibody treatments, respectively. | ||
| Vaccine effectiveness | Pfizer, Moderna, and Janssen are effective | Potential reduction in neutralization by post-vaccination sera | Minimal impact on neutralization by post vaccination sera for B.1.1.7 variant, while other variants have moderate reduction in neutralization by post-vaccination sera. | Significant impact on neutralization by post vaccination sera |
| Notification to WHO and CDC | No | No | Yes | Yes |