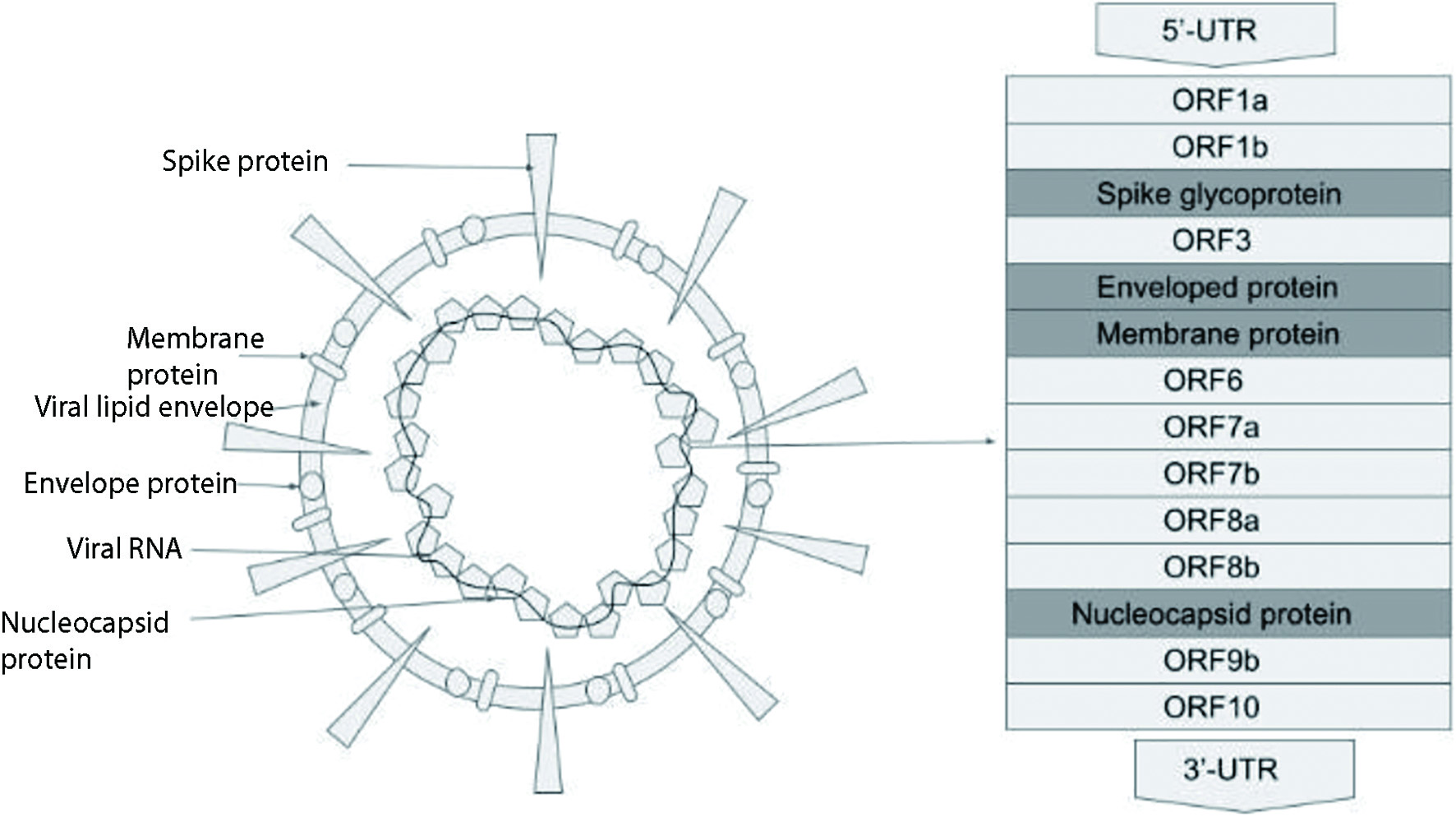
Figure 1. COVID-19 viral structure. COVID-19: coronavirus disease 2019.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 13, Number 4, April 2021, pages 204-213
Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use
Figures

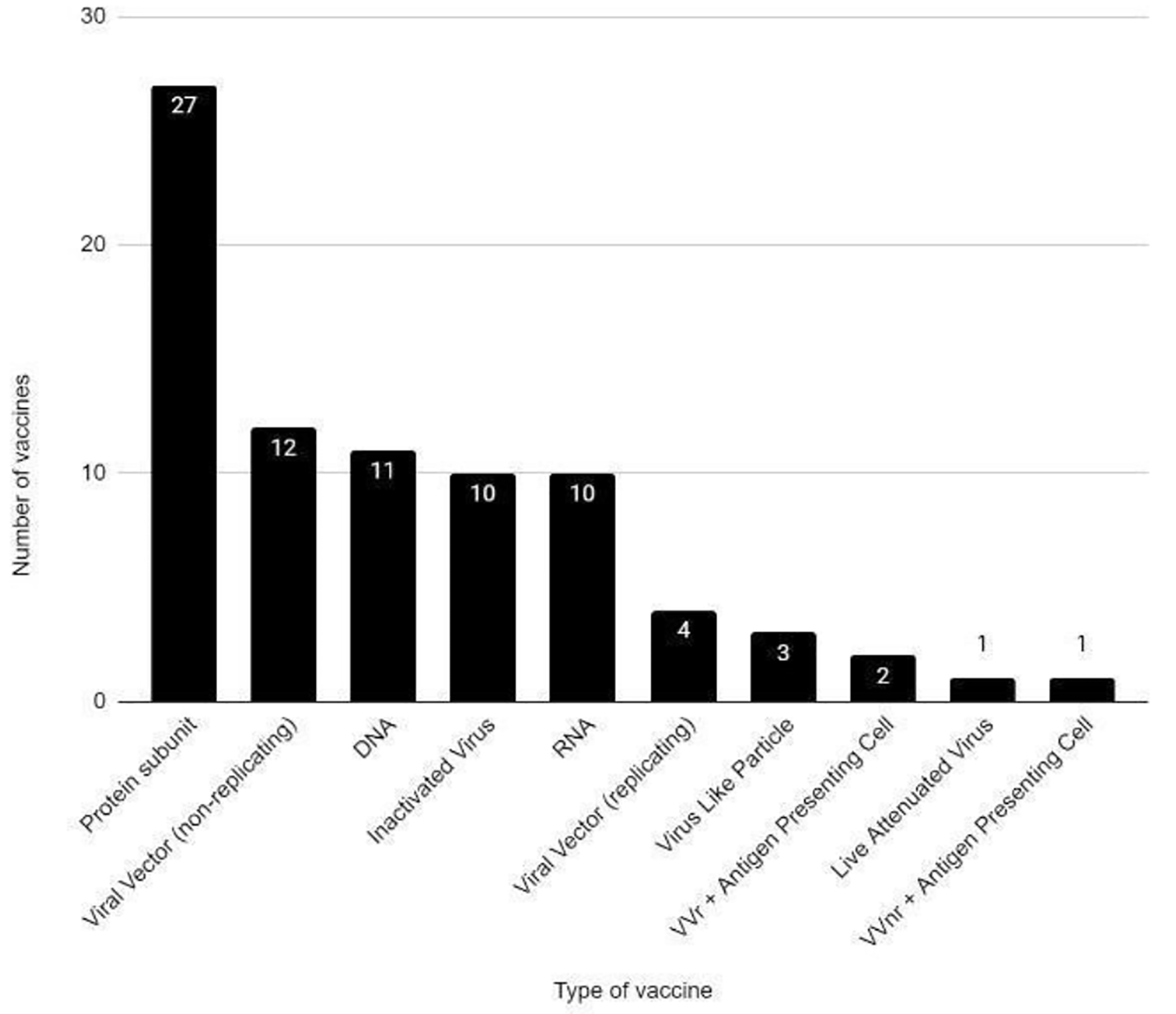
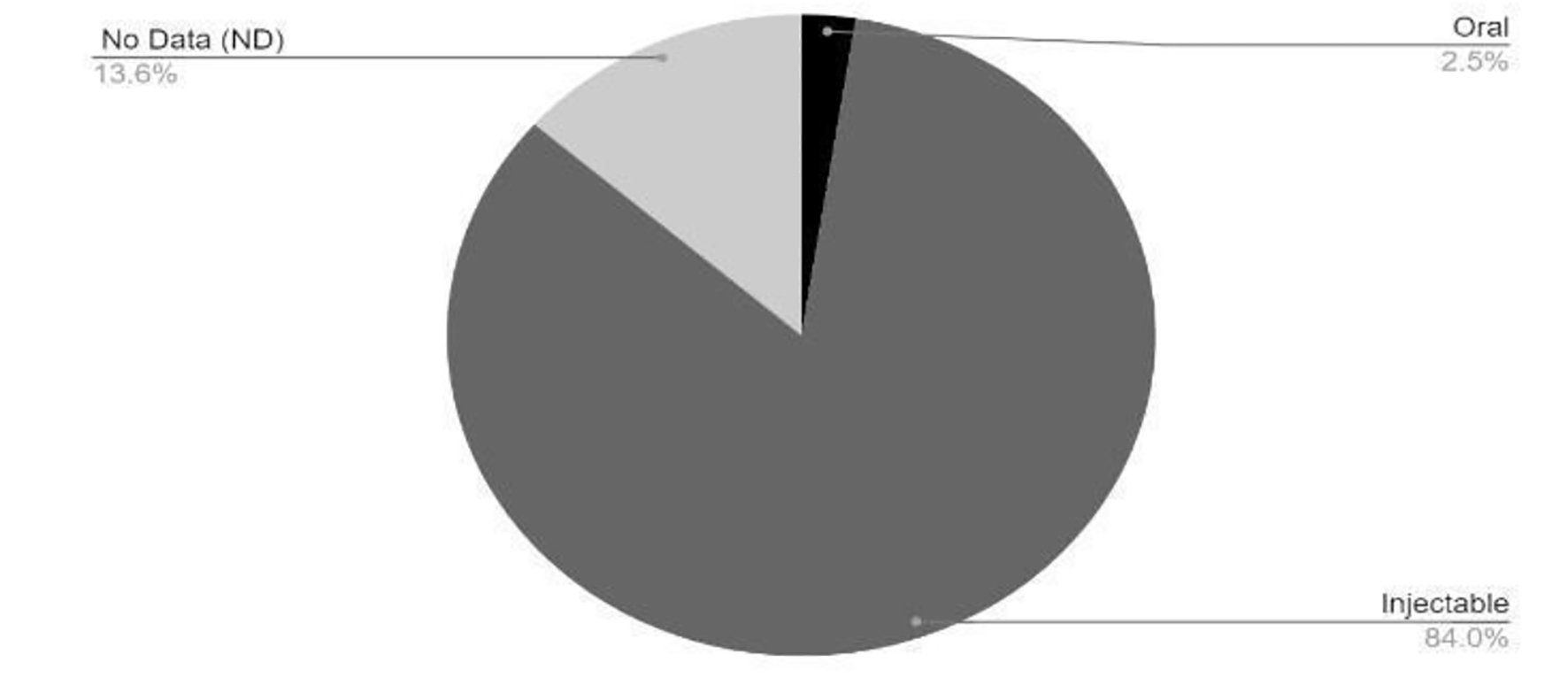
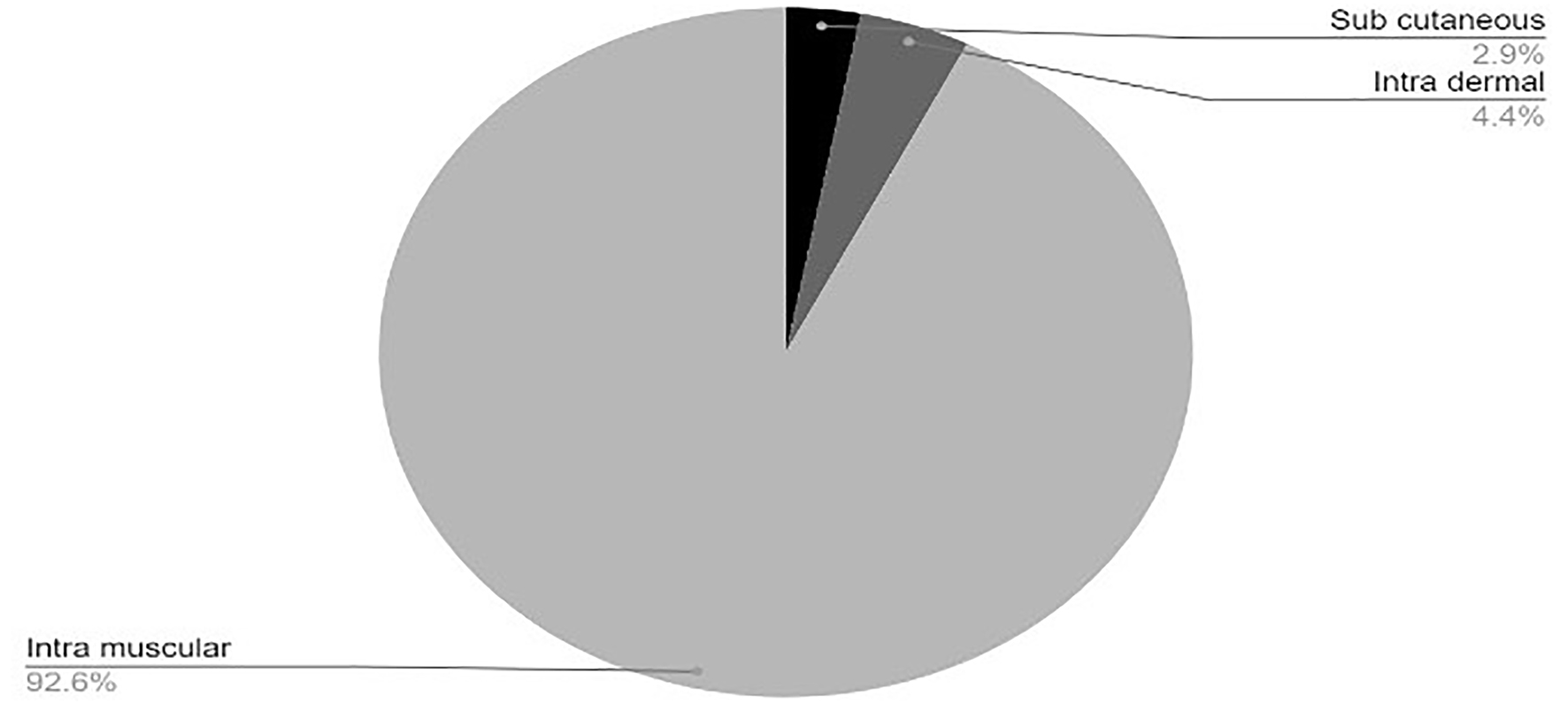
Tables
| Country of development | Developer | Type of vaccine | Usage |
|---|---|---|---|
| USA: United States of America; UK: United Kingdom; EU: European Union; UAE: United Arab Emirates. | |||
| USA/Germany | Pfizer-BioNTech | mRNA | Approved in several countries. Emergency use in USA, EU, UK, Bahrain, Canada, Saudi Arabia, Mexico, etc. |
| USA | Moderna | mRNA | Approved in Switzerland. Emergency use in USA, UK and EU |
| Russia | Gamaleya | Viral vector | Early use in Russia |
| UK/Sweden | Oxford-AstraZeneca | Viral vector | Emergency use in UK and EU |
| China | CanSino | Viral vector | Approved in China |
| USA/Belgium | Johnson and Johnson | Viral vector | Emergency use in USA and Bahrain |
| Russia | Vector Institute | Protein subunit | Early use in Russia |
| China | Sinopharm | Inactivated virus | Approved in China, UAE and Bahrain. Emergency use in Egypt |
| China | Sinovac | Inactivated virus | Approved in China. Emergency use in Brazil |
| China | Sinopharm-Wuhan | Inactivated virus | Limited use in China and UAE |
| India | Bharat biotech | Inactivated virus | Emergency use in India |
| COVID-19 vaccine | Common side effects | Other adverse effects |
|---|---|---|
| COVID-19: coronavirus disease 2019. | ||
| Pfizer-BioNTech | Fever, cough, fatigue, headache, shortness of breath, chills, muscle pain, sore throat, diarrhea, or vomiting, local injection site redness or swelling. | Lymphadenopathy, Bell’s palsy, paroxysmal ventricular arrhythmia, right leg paresthesia and shoulder injury. |
| Moderna | Fever, local pain, swelling, tenderness and erythema at the injection site. Axillary lymphadenopathy, fatigue, headache, myalgia, arthralgia, chills and nausea/vomiting. | Hypersensitivity reactions, Bell’s palsy and some other lymphadenopathy. |
| Janssen | Mild to moderate febrile episode, injection site pain, fatigue, headache and myalgia. | |