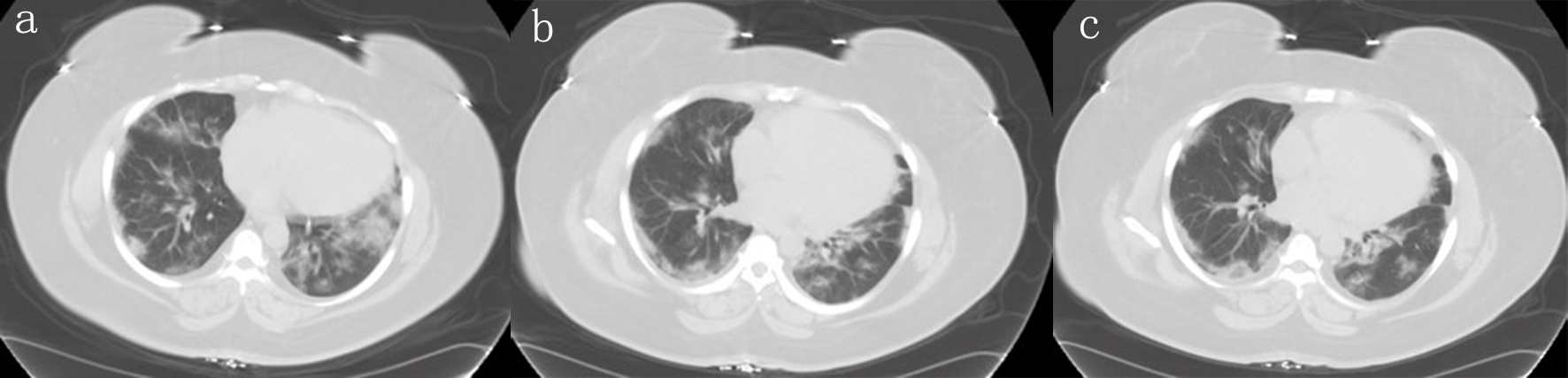
Figure 1. Different planes of the CT scan showing bilateral worsening of ground-glass opacities at bases. CT: computed tomography.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 12, Number 5, May 2020, pages 315-319
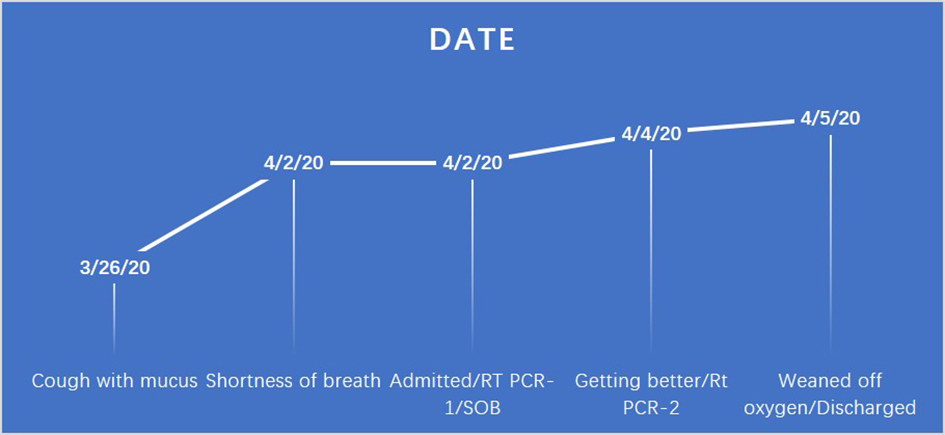
A Comprehensive Approach Is Vital for Diagnosing COVID-19: A Case of False Negative
Figures

Tables
| Laboratory findings | Patients value (normal values) |
|---|---|
| CRP: C-reactive protein; LDH: lactate dehydrogenase; INR: international normalized ratio; BMI: body mass index. | |
| White blood cell count | 8.0 (4.8 - 10.8 × 109/L) |
| Absolute lymphocyte count | 1.5 (1.0 - 3.5 × 109/L) |
| Procalcitonin | < 0.05 (< 0.05) |
| D-dimer | 186 (< 255 ng/mL) |
| CRP (high) | 13.2 (0.000 - 0.744 mg/dL) |
| LDH (high) | 390 (100 - 235 U/L) |
| Serum ferritin | 284 (11 - 307 ng/mL) |
| Erythrocyte sedimentation rate (high) | 59 (0 - 20 mm/h) |
| Respiratory viral panel | Negative |
| Vitamin D (low) | 17 (> 30) |
| INR (high) | 1.3 (0.9 - 1.2) |
| BMI | 42.91 kg/m2 |
| Methods of testing | What the test interprets | Turnaround time |
|---|---|---|
| ELISA: enzyme-linked immunosorbent assay; RDT: rapid diagnostic test. | ||
| Neutralization assay | Tests to look for active antibodies in subject serum which can inhibit virus growth ex vivo. Indicates if the patient is protected against future infection. | 3 - 5 days |
| ELISA | Quantify the presence or absence of antibodies against the virus in the subject’s serum. | 1 - 5 h |
| RDT | Qualitatively tests for the presence or absence of antibodies against virus in the subject’s serum. Cannot quantify the antibody titer. | 10 - 30 min |
| Company/method of testing | Country of development | Sensitivity and specificity of the test |
|---|---|---|
| ELISA: enzyme-linked immunosorbent assay; COVID-19: coronavirus disease 2019; FDA: Food and Drug Administration; IgG: immunoglobulin G; RDT: rapid diagnostic test. | ||
| Mount Sinai laboratory COVID-19 ELISA IgG antibody test/ELISA | USA | Not available |
| VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack/Total Calibrator (Ortho Clinical Diagnostics)/Modified ELISA | USA | Sensitivity: 83%, specificity: 100% |
| Cellex/RDT | USA/China | Sensitivity: 93.8%, specificity: 95.6% |
| ChemBio/RDT | USA | Not available |
| Epitope Diagnostics, Ltd/ELISA | USA | Not available |
| BioMedomics/RDT for research use only | USA | Sensitivity: 88.66%, specificity: 90.63% |
| Ray Biotech/RDT for research use only | USA | Not available |
| Emory University/ELISA for research use only | USA | Not available |