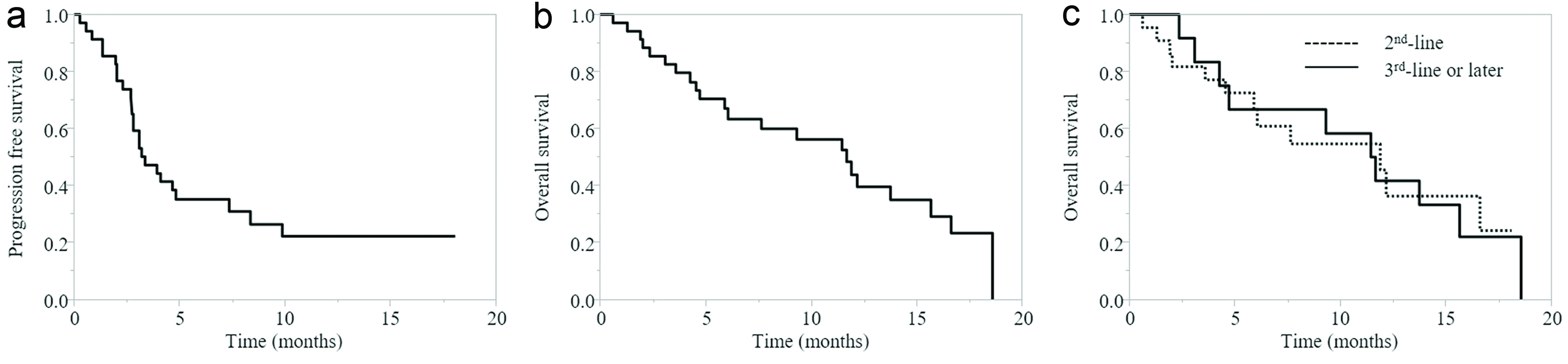
Figure 1. (a) PFS of all cases. (b) OS of all cases. (c) OS according to pembrolizumab treatment line. PFS: progression-free survival; OS: overall survival.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 12, Number 5, May 2020, pages 300-306
Treating Japanese Patients With Pembrolizumab for Platinum-Refractory Advanced Urothelial Carcinoma in Real-World Clinical Practice
Figure

Tables
| Characteristic (n = 34) | |
|---|---|
| ECOG-PS: Eastern Cooperative Oncology Group Performance Status; UC: urothelial carcinoma; Hb: hemoglobin; NLR: neutrophil/lymphocyte ratio; LDH: lactate dehydrogenase; CRP: C-reactive protein. | |
| Age (years), median (range) | 71 (57 - 87) |
| Male sex, n (%) | 28 (82) |
| ECOG-PS score, n (%) | |
| 0 | 20 (59) |
| ≥ 1 | 14 (41) |
| Primary tumor site, n (%) | |
| Bladder | 13 (38) |
| Upper urinary tract | 12 (35) |
| Bladder + upper urinary tract | 9 (27) |
| Pure UC in histologic testing, n (%) | 27 (79) |
| Number of prior chemotherapy regimens | |
| 1 | 22 (64) |
| 2 | 6 (18) |
| 3 | 6 (18) |
| Hb < 10g/dL, n (%) | 14 (41) |
| NLR, median (range) | 2.2 (0.7 - 18.0) |
| Albumin < 4.1 g/dL, n (%) | 23 (68) |
| LDH > 222 U/L, n (%) | 10 (29) |
| CRP > 0.14 mg/dL, n (%) | 26 (76) |
| Visceral metastases, n (%) | 27 (79) |
| Liver metastases, n (%) | 11 (32) |
| Time from previous chemotherapy < 3 months, n (%) | 27 (79) |
| Any grade | Grade 3 | |
|---|---|---|
| AEs: adverse events. | ||
| Treatment-related AEs, n (%) | 21 (62) | 8 (24) |
| Fatigue | 6 | 2 |
| Pruritus | 5 | 0 |
| Fever | 2 | 0 |
| Hematuria | 2 | 2 |
| Anorexia | 2 | 1 |
| Lung infection | 2 | 1 |
| Pain | 1 | 1 |
| Anemia | 1 | 1 |
| Kidney infection | 1 | 1 |
| Creatinine increased | 1 | 1 |
| Aspartate and alanine aminotransferase increased | 1 | 1 |
| Constipation | 1 | 0 |
| Infusion related reaction | 1 | 0 |
| Diarrhea | 1 | 0 |
| Ileus | 1 | 0 |
| Interstitial pneumonia | 1 | 0 |
| Hyponatremia | 1 | 0 |
| Eyelid function disorder | 1 | 0 |
| Nervous system disorders | 1 | 0 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| HR: hazard ratio; CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; UC: urothelial carcinoma; Hb: hemoglobin; NLR: neutrophil/lymphocyte ratio; LDH: lactate dehydrogenase; CRP: C-reactive protein. | ||||
| Age | 0.99 (0.93 - 1.05) | 0.72 | ||
| Sex | ||||
| Male | 1 | |||
| Female | 0.91 (0.33 - 2.52) | 0.86 | ||
| ECOG -PS | ||||
| 0 | 1 | 1 | ||
| 1 | 2.81 (1.13 - 7.29) | 0.03 | 1.61 (0.58 - 4.45) | 0.36 |
| Primary tumor site | ||||
| Bladder | 1 | |||
| Upper urinary tract | 0.95 (0.34 - 2.70) | 0.93 | ||
| Bladder + upper urinary tract | 1.69 (0.56 - 5.11) | 0.35 | ||
| Histology | ||||
| Pure UC | 1 | |||
| Mixed UC | 2.84 (0.66 - 12.25) | 0.16 | ||
| Hb | ||||
| ≥ 10 g/dL | 1 | |||
| < 10 g/dL | 1.50 (0.61 - 3.58) | 0.37 | ||
| NLR | 1.24 (1.03 - 1.46) | 0.03 | 1.07 (0.86 - 1.30) | 0.50 |
| Albumin | ||||
| ≥ 4.1 g/dL | 1 | |||
| < 4.1 g/dL | 1.57 (0.61 - 4.09) | 0.34 | ||
| LDH | ||||
| ≤ 222 U/L | 1 | |||
| > 222 U/L | 3.76 (1.41 - 10.03) | 0.07 | ||
| CRP | ||||
| ≤ 0.14 mg/dL | 1 | |||
| > 0.14 mg/dL | 2.04 (0.68 - 6.09) | 0.20 | ||
| Liver metastases | ||||
| Negative | 1 | 1 | ||
| Positive | 3.61 (1.47 - 8.84) | < 0.01 | 4.23 (1.48 - 12.08) | < 0.01 |
| Time from previous chemotherapy | ||||
| < 3 months | 1 | 1 | ||
| ≥ 3 months | 3.59 (1.26 - 10.22) | 0.02 | 5.06 (1.43 - 17.91) | 0.01 |