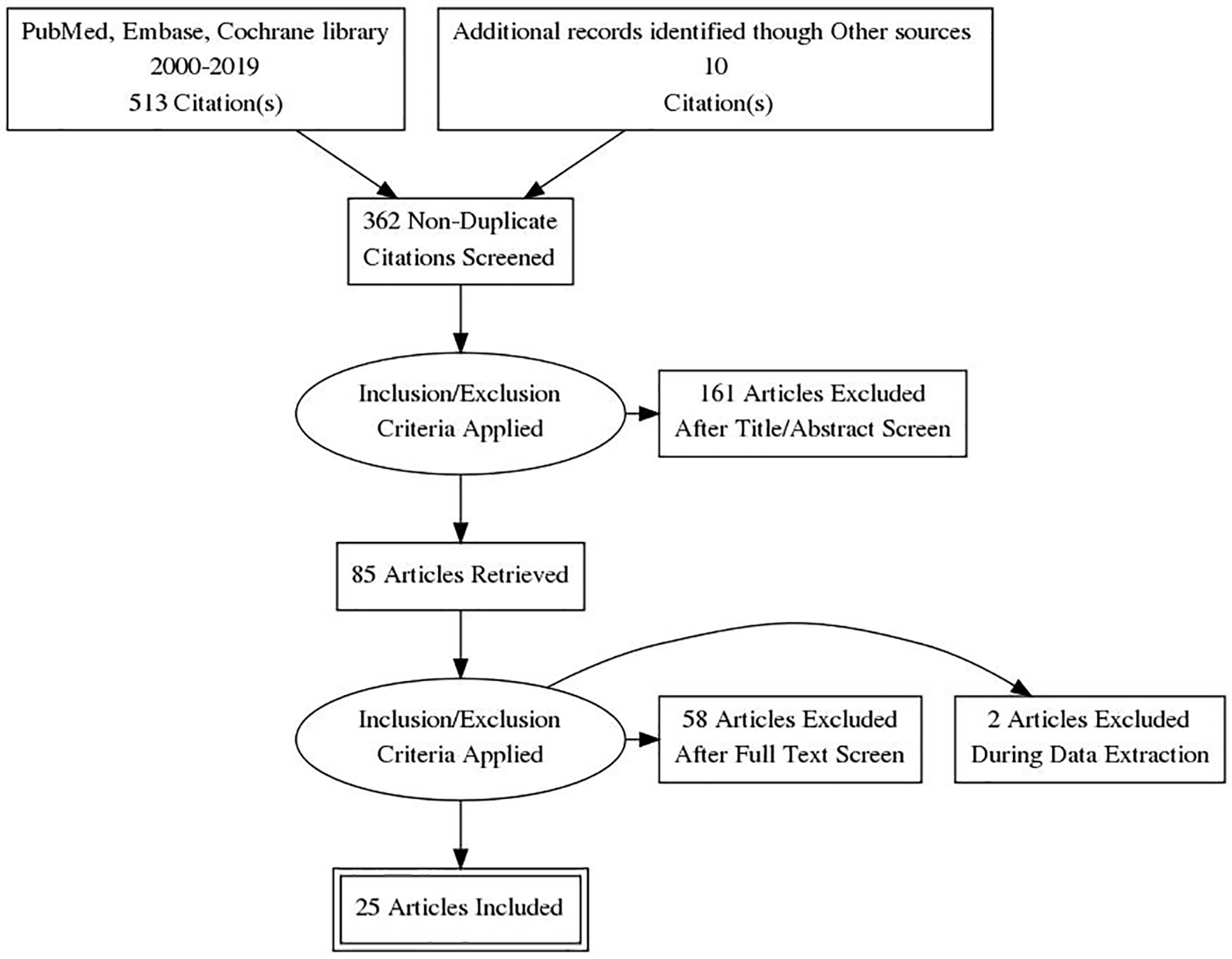
Figure 1. PRISMA flow diagram of literature search and selection process. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 12, Number 3, March 2020, pages 129-141
Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials
Figure

Tables
| Author, year of publication | Population (no. of participants) | Study design | CBD dose and scheme | Formulation/route | Primary outcome | Measures | Effect |
|---|---|---|---|---|---|---|---|
| aActive-controlled trial, amisulpride. bConcomitant treatment. cCBD-rich botanical extracts capsules contained other compound (up to 4.7% THC). IR: individually randomized; NR: non-randomized; CR: cluster randomized; CBD: cannabidiol; HDL: high-density lipoprotein. | |||||||
| Anxiety | |||||||
| Bergamaschi et al (2011) [17] | Social anxiety disorder patients (24) | IR parallel group trial | 600 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale (sub-scales; anxiety, cognitive impairment, discomfort and alert) | CBD significantly reduced anxiety, cognitive impairment, discomfort and alert during simulation public speaking test |
| Crippa et al (2011) [22] | Social anxiety disorder patients (10) | IR cross-over trial | 400 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale | Acute administration reduced subjective anxiety |
| Arndt et al (2017) [25] | Healthy volunteers (38) | IR cross-over trial | 300, 600 and 900 mg; single dose | Oral solution | Reactivity to negative stimuli | Behavioral tasks | Single doses of CBD had little effect on reactions to negative emotional stimuli |
| Crippa et al (2004) [21] | Healthy volunteers (10) | IR cross-over trial | 400 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale (sub-scales; anxiety, physical sedation, mental sedation and other feelings and attitudes) | CBD significantly decreased subjective anxiety and increased mental sedation |
| Zuardi et al (2017) [19] | Healthy volunteers (60) | IR parallel group trial | 100, 300 and 900 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale (anxiety and sedation factors) | CBD 300 mg reduced subjective anxiety measures and presented lower sedation level when compared with clonazepam. This was not observed with CBD 100 and 900 mg. |
| Martin-Santos et al (2012) [23] | Healthy volunteers (16) | NR controlled trial | 600 mg; single dose | Oral | Anxiety | Spielberger State Anxiety Inventory, visual analogue mood scale | There was no difference between CBD and placebo on anxiety levels. |
| Bhattacharyya et al (2010) [24] | Healthy volunteers (15) | NR controlled trial | 600 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale- tranquilization and calming sub-scale. | CBD single dose reduced anxiety levels. |
| Linares et al (2019) [18] | Healthy volunteers (57) | IR parallel group trial | 150, 300 and 600 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale | 300 mg of CBD significantly reduced anxiety during simulation public speaking test |
| Das et al (2013) [27] | Healthy volunteers (48) | IR parallel group trial | 32 mg; single dose | Inhalation/vaporized | Extinction and consolidation | Skin conductance and shock expectancy measures | CBD enhanced consolidation of extinction learning |
| Hindocha et al (2015) [26] | High and low cannabis use and high and low schizotipy (48) | CR cross-over trial | 16 mg; single dose | Inhalation/vaporized | Emotional processing | Computer-based emotional processing task | CBD improved recognition of emotional facial affect. |
| Hundal et al (2018) [20] | Non-clinical high paranoid group (32) | IR parallel group trial | 600 mg; single dose | Oral capsules | Anxiety | Beck’s anxiety inventory | CBD apparently seemed to increase anxiety levels. |
| Psychotic disorders | |||||||
| Hallak et al (2010) [31] | Schizophrenia patients (28) | IR parallel group trial | 300 or 600 mg; single dose | Oral capsules | Cognitive functioning | Stroop color word test | 300 and 600 mg of CBD do not lead to cognitive improvement |
| Boggs et al (2018) [28] | Chronic schizophrenia patients (42) | IR parallel group trial | 600 mg/day; 6 weeks | Oral capsules | Psychotic symptoms, cognitive functioning | Positive and negative syndrome scale T score of MATRICS consensus cognitive battery | Psychotic symptoms improved in both groups without significant difference, cognitive functioning improved only in placebo group. |
| Leweke et al (2012) [29] | Acutely psychotic patients (32) | IR parallel group triala | 200 mg/day up to 800 mg/day; 4 weeks | Oral capsules | Psychotic symptoms | Positive and negative syndrome scale; brief psychiatric rating scale | CBD is as effective as amisulpride in improving psychotic symptoms |
| McGuire et al (2018) [30] | Schizophrenia patients (88) | IR parallel group trial | 1,000 mg/day; 6 weeksb | Oral solution | Psychotic symptoms | Positive and negative syndrome scale, clinical global impressions scale | Treatment with CBD improved positive psychotic symptoms and clinicians’ impressions of illness improvement. |
| Cognitive functioning, level of functioning | Brief assessment of cognition in schizophrenia; global assessment of functioning scale | Improvement in cognitive performance and in the level of overall functioning with CBD although does not reach statistical significance. | |||||
| Hundal et al (2018) [20] | Non-clinical high paranoid group (32) | IR parallel group trial | 600 mg; single dose | Oral capsules | Persecutory ideation | State social paranoia scale, community assessment of psychic experiences scale | CBD had no effect on precursory thinking and psychotic-like experiences |
| Cannabis use disorder | |||||||
| Haney et al (2016) [32] | Healthy cannabis smokers (32) | IR cross-over trial | 200, 400 and 800 mg; single dose | Oral capsules | Cannabis subjective effects | Subjective mood and drug effects measured with visual analogue scale | CBD did not alter the subjective effects of smoked cannabis |
| Nicotine addiction | |||||||
| Morgan et al (2013) [33] | Tobacco smokers (24) | IR parallel group trial | 400 µg; 1 week | Inhalation/vaporized | Reduction in the number of cigarettes smoked | Number of cigarettes smoked | CBD reduced the number of cigarettes smoked during treatment and at follow-up |
| Hindocha et al (2018) [34] | Tobacco smokers (30) | IR parallel group trial | 800 mg; single dose | Oral capsules | Nicotine withdrawal | Visual probe task and pleasantness rating task | CBD reduced the salience and pleasantness of cigarette cues, compared with placebo but did not influence tobacco craving or withdrawal |
| Dyslipidemia | |||||||
| Jadoon et al (2016) [35] | Patients with type 2 diabetes and dyslipidemia (62) | IR parallel group trial | 200 mg/day; 13 weeks | Oral capsules | HDL cholesterol concentrations | Enzymatic calorimetric assays | CBD did not produce any effect on HDL levels compared to placebo |
| Crohn’s disease | |||||||
| Naftali et al (2017) [36] | Patients with diagnosis of Crohn’s disease (19) | IR parallel group trial | 20 mg/day; 8 weeksb | Sublingual oil | Disease activity | Crohn’s disease activity index | A reduction in disease activity at the end of the study but no significant difference with placebo. |
| Ulcerative colitis | |||||||
| Irving et al (2018) [37] | Patients with mild to moderate ulcerative colitis (60) | IR parallel group trial | 50 mg up to 250 mg/day; 10 weeksa, b, c | Oral capsules | Remission at the end of treatment | Mayo score of ≤ 2 | The primary endpoint was negative but CBD may be beneficial for symptomatic treatment of ulcerative colitis |
| Author/year of publication | Indication | No. of participants | Dose evaluated (administration scheme) | Administration method | Follow-up | Outcome | Measure | Effect |
|---|---|---|---|---|---|---|---|---|
| aCortex Technologies, Hadsund, Denmark. bDelfin Technologies, Kuopio, Finland. cConcomitant treatment for the condition with anti-oxidant and pain killer. dConcomitant treatment with anti-seizure drugs. N/A: non-available; HPV: human papillomavirus; ADR: adverse drug reaction. | ||||||||
| Szaflarski et al (2018) [38] | Epilepsy | 60 | 5 up to 50 mg/kg/dayd | Oral oil | 48 weeks | Seizure control | Seizure frequency, Chalfont seizure severity scale | Improved compared to baseline |
| Palmieri et al (2019) [39] | Skin disorders | 20 | N/A (two times a day/3 months) | Topical gel | 3 months | Hydration | Hydration probe | Improved compared to baseline |
| Transepidermal water loss | DermaLab® USB instrumenta | Improved compared to baseline | ||||||
| Skin elasticity | ElastiMeter®b | Improved compared to baseline | ||||||
| Severity of atopic dermatitis | Scoring atopic dermatitis | Improved compared to baseline | ||||||
| Severity of acne | Acne disability index | Improved compared to baseline | ||||||
| Severity of psoriasis | Psoriasis area severity index | Improved compared to baseline | ||||||
| Palmieri et al (2017) [40] | ADR following HPV vaccine | 12 | 25 mg/mL, 150 mg/mL (one time/day × 3 months)c | Sublingual oil drops | 3 months | Quality of life | Medical outcome short-form health survey questionnaire | Improved compared to baseline |
| Outcomes | No. of participants (studies) follow-up | Certainty of the evidence (GRADE)a | Impact |
|---|---|---|---|
| The outcome of interest are: anxiety symptoms, psychotic symptoms and cognitive function. A pooled effect estimate was not available and a narrative synthesis of the evidence was provided. aSymbols are used to describe certainty in evidence profiles. High certainty: ⨁⨁⨁⨁; Moderate certainty: ⨁⨁⨁◯; Low certainty: ⨁⨁◯◯; Very low certainty: ⨁◯◯◯. RCT: randomized controlled trial. | |||
| Anxiety symptoms assessed with visual analogue mood scale | 44 (two RCTs) | ⨁⨁◯◯ low | All studies showed a reduction in symptoms compared to placebo. |
| Psychotic symptoms assessed with positive and negative syndrome scale, brief psychiatric rating scale | 171 (three RCTs) | ⨁⨁⨁◯ moderate | Two studies showed improvement in psychotic symptoms. Some variability in the intervention (doses) and population (stage of illness) was noted. |
| Cognitive function assessed with T score of MATRICS consensus cognitive battery, stroop color word test, composite score on the brief assessment of cognition in schizophrenia | 148 (three RCTs) | ⨁⨁⨁◯ moderate | Two studies showed no improvement in cognitive function. One showed only small improvements. |
| Follow-up < 1 day | Follow-up 1 week | Follow-up 10 weeks | Follow-up 13 weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Cannabidiol (%) | Placebo (%) | Cannabidiol (%) | Placebo (%) | Cannabidiol (%) | Placebo (%) | Cannabidiol (%) | Placebo (%) | |
| Subjects experiencing any TEAE (%) | 66.7 | 62.5 | 100 | 83.3 | 90 | 48 | 84.6 | 92.9 |
| Nervous system disorders | 33.3 | 37.5 | 66.7 | 33.3 | 83 | 26 | ||
| Gastrointestinal system disorders | 16.7 | 50.0 | 66.7 | 50.0 | 38 | 16 | ||
| Infections and infestations | 22.2 | 0 | 0 | 0 | ||||
| Psychiatric disorders | 0 | 0 | 24 | 3 | ||||
| General disorders and administration site conditions | 22.2 | 50.0 | 21 | 10 | ||||
| Musculoskeletal and connective tissue disorders | 0 | 16.7 | 14 | 0 | ||||
| Skin and subcutaneous tissue disorders | 2.2 | 16.7 | 3 | 0 | ||||