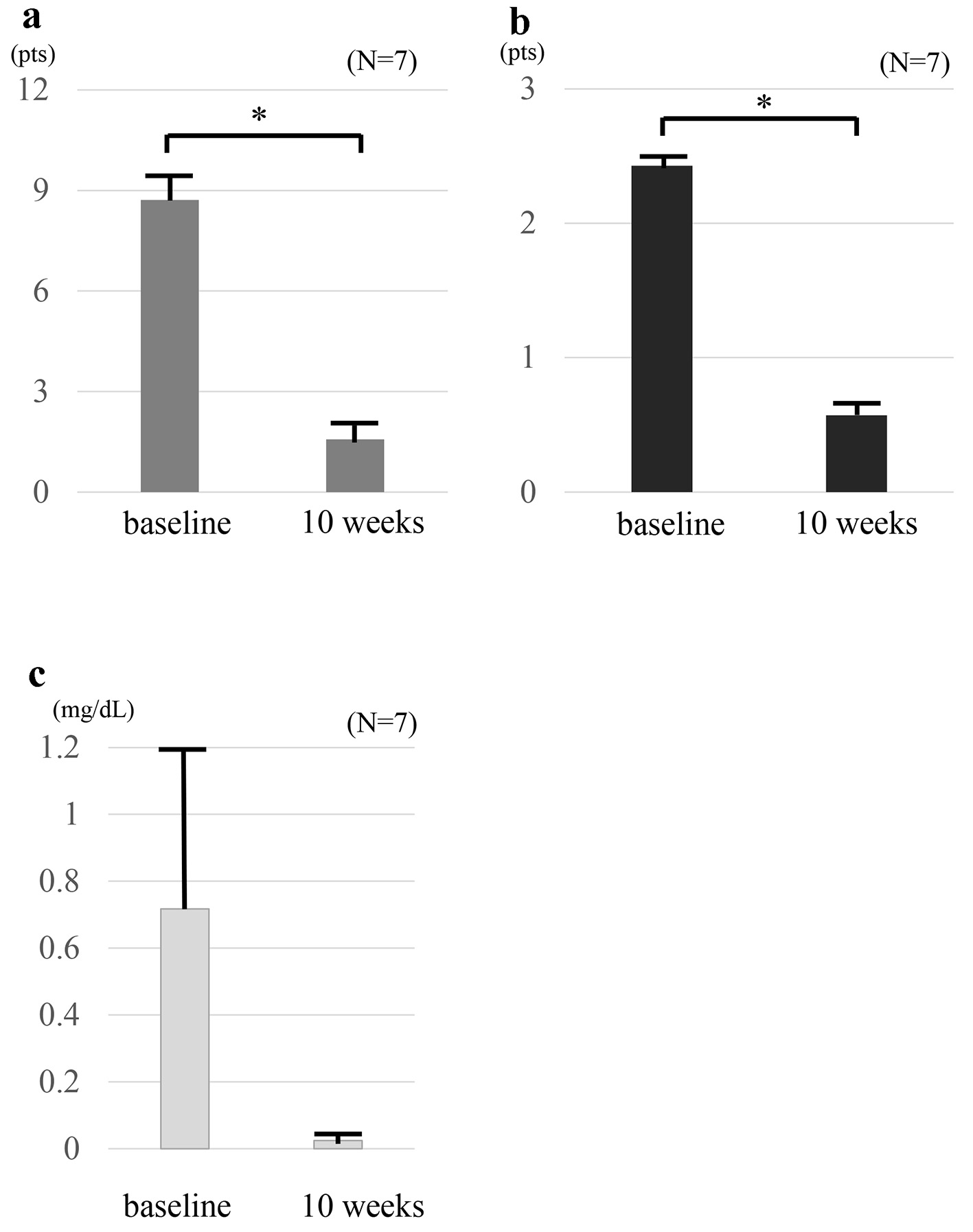
Figure 1. Clinical outcomes at baseline and at 10 weeks in seven patients who received combination therapy TOF and intensive GMA. The mean full Mayo scores (a) and endoscopic sub-scores (b) show significant differences between at baseline and 10 weeks (P < 0.01). The mean full Mayo scores decreased from 8.71 ± 0.80 at baseline to 1.57 ± 0.48 at 10 weeks, and the mean endoscopic subscore decreased from 2.4 ± 0.2 at baseline to 0.6 ± 0.2 at 10 weeks. CRP values (c) show no significant difference between at baseline and 10 weeks (P = 0.20). The mean CRP level decreased from 0.71 ± 0.49 mg/dL at baseline to 0.02 ± 0.005 mg/dL at 10 weeks. The values are presented as means ± standard error values and were made by using paired t-test. *P< 0.01. TOF: tofacitinib; GMA: granulocyte and monocyte adsorptive apheresis; CRP: C-reactive protein.