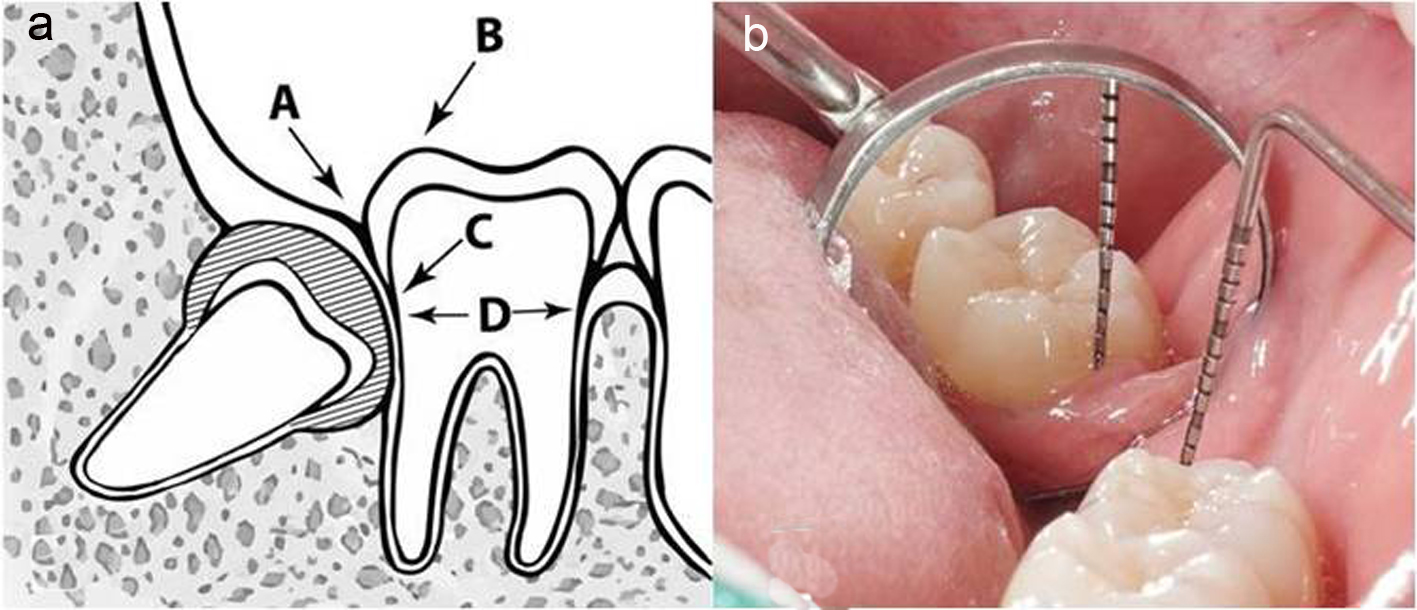
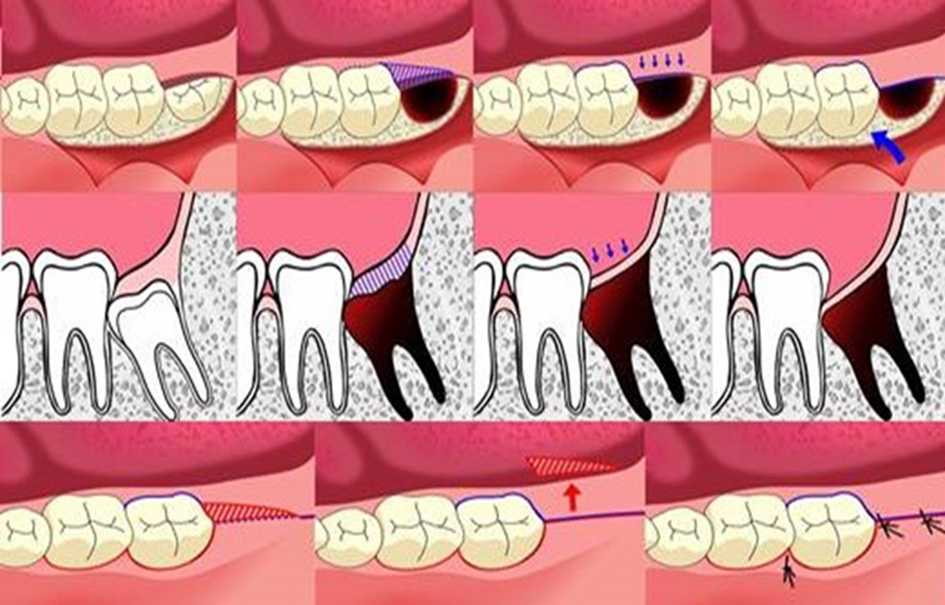
| Age between 18 - 25 years; having good systemic health; no smoking; having symmetrically positioned MTMI on both sides; not allergic to 4% articaine with 1:100,000 adrenaline; understanding and carrying out the instructions given by the investigators; having given written informed consent for the study | Pregnant or lactating mother; having serious medical conditions such as hypertension; cardiovascular problems, renal and/or liver failure, or smoking habit; inability to attend the periodic examinations; taking any medication during the previous 5 days prior to the surgery that would alter their pain perception (e.g. analgesics, antidepressants) | Patients could leave the study at any time depending on their own decisions and will |