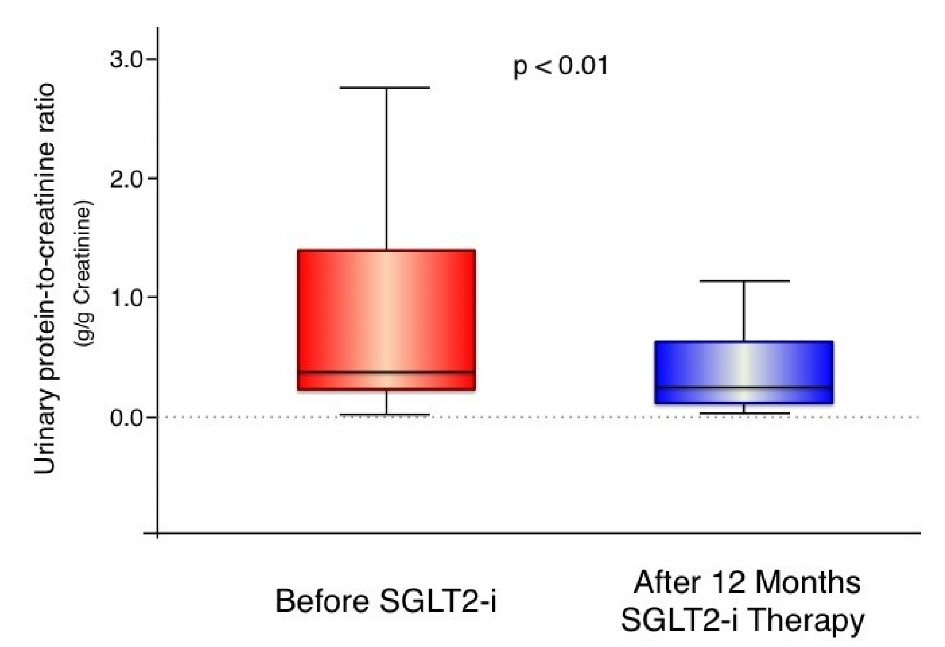
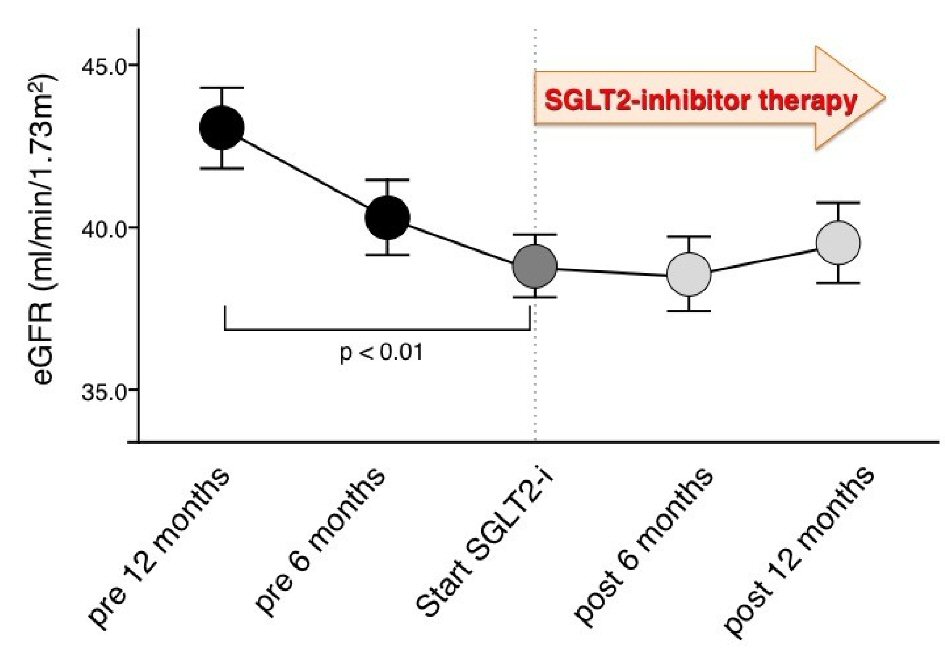
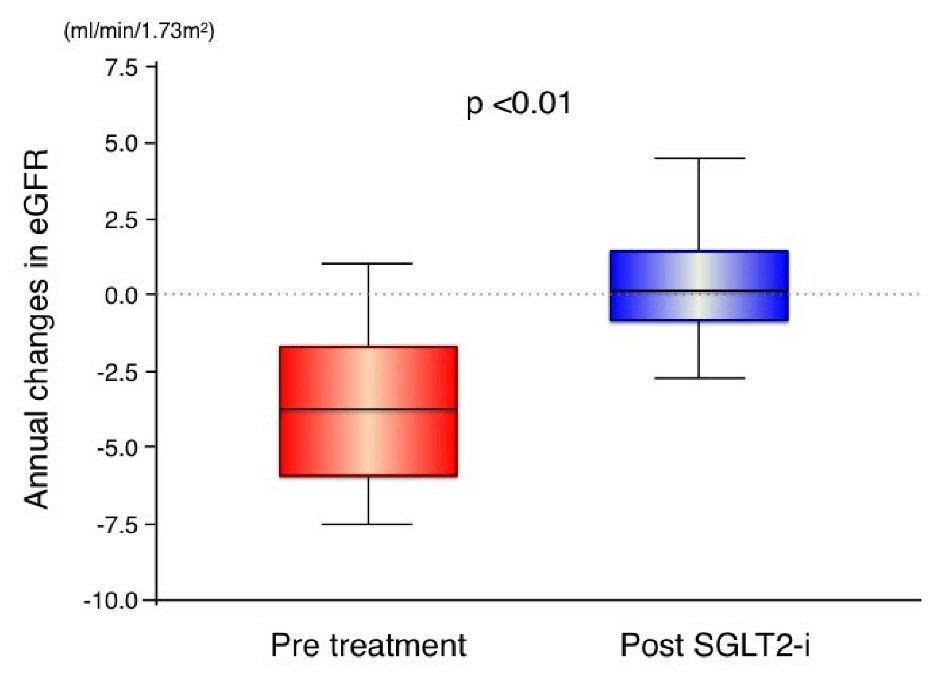
| Age (years) | 64.8 ± 9.8 |
| Sex: male (%) | 30 (71.4) |
| Body mass index (kg/m2) | 28.2 ± 3.6 |
| Hypertension (%) | 40 (95.2) |
| Dyslipidemia (%) | 40 (95.2) |
| Current smoking (%) | 9 (21.4) |
| Retinopathy (%) | 26 (61.9) |
| Cerebrovascular/cardiovascular diseases (%) | 10 (23.8) |
| Duration of diabetes (years) | 18.0 ± 7.4 |
| Hemoglobin A1c (%) | 7.6 (7.1 - 8.2) |
| Fasting plasma glucose (mg/dL) (n = 21) | 158 (129 - 193) |
| Anti-diabetic drugs | - |
| Sulfonylureas (%) | 9 (21.4) |
| Glinide (%) | 1 (2.4) |
| Metformin (%) | 21 (50.0) |
| Alpha-glucosidase inhibitor (%) | 8 (19.0) |
| Thiazolidinedione (%) | 5 (11.9) |
| Dipeptidyl peptidase-4 inhibitor (%) | 10 (23.8) |
| Glucagon like peptide-1 receptor agonist (%) | 9 (21.4) |
| Insulin (%) | 30 (71.4) |
| Additional SGLT2i (Dapa/Empa/Cana/Luse/Tofo) (%) | 33.0/19.0/4.8/31.0/11.9 |
| Treatment duration of SGLT2i (months) | 21.5 (15.0 - 31.0) |
| Treatment with ACEI or ARB (%) | 30 (71.4) |
| Treatment with diuretics including aldosterone antagonist (%) | 12 (28.6) |
| eGFR (mL/min/1.73m2) | 40.4 (36.5 - 43.0) |
| Chronic kidney disease: stage-4 (eGFR< 30 mL/min/1.73m2) (%) | 4 (9.5) |
| Qualitative assessment of urinary protein levels (%) | - |
| Negative (-) (< 15 mg/dL) | 15 (35.7) |
| Borderline (±) (15 - 30 mg/dL) | 8 (19.0) |
| Mild (+) (30 - 100 mg/dL) | 6 (14.3) |
| Moderate (++) (100 - 300 mg/dL) | 8 (19.0) |
| Severe (+++) (> 300 mg/dL) | 5 (11.9) |