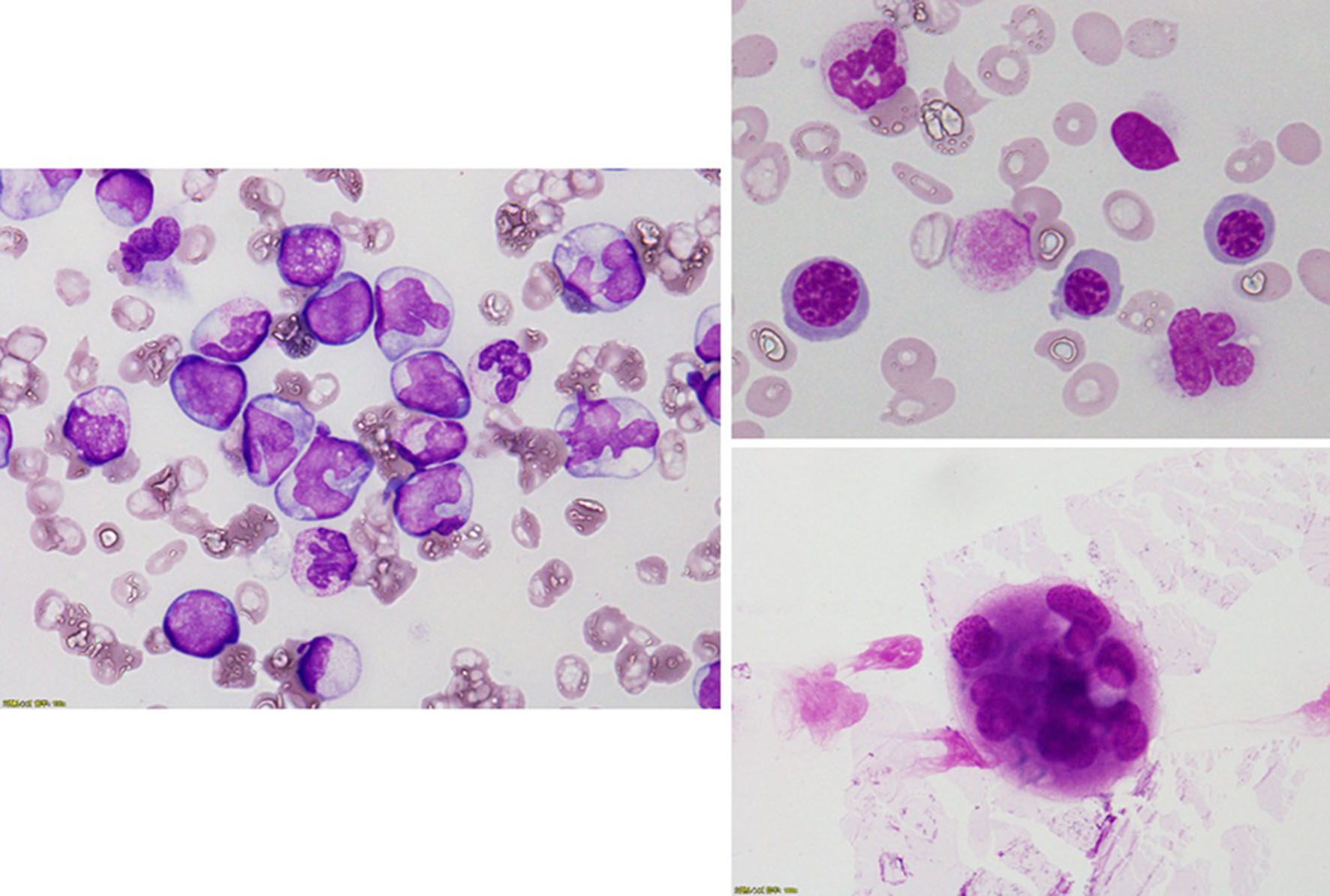
Figure 1. Bone marrow examination revealed a hypercellular bone marrow with decreased megakaryocytes and increased monocytes. Forty percent of megakaryocytes had multiple, widely-separated nuclei and 10% of erythrocytes had megaloblastoid change.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 11, Number 2, February 2019, pages 145-150
Massive Hemoptysis Due to the Rupture of Thoracic Aortic Aneurysm Caused by Leukemic Cell Infiltration in a Patient With Chronic Myelomonocytic Leukemia
Figures

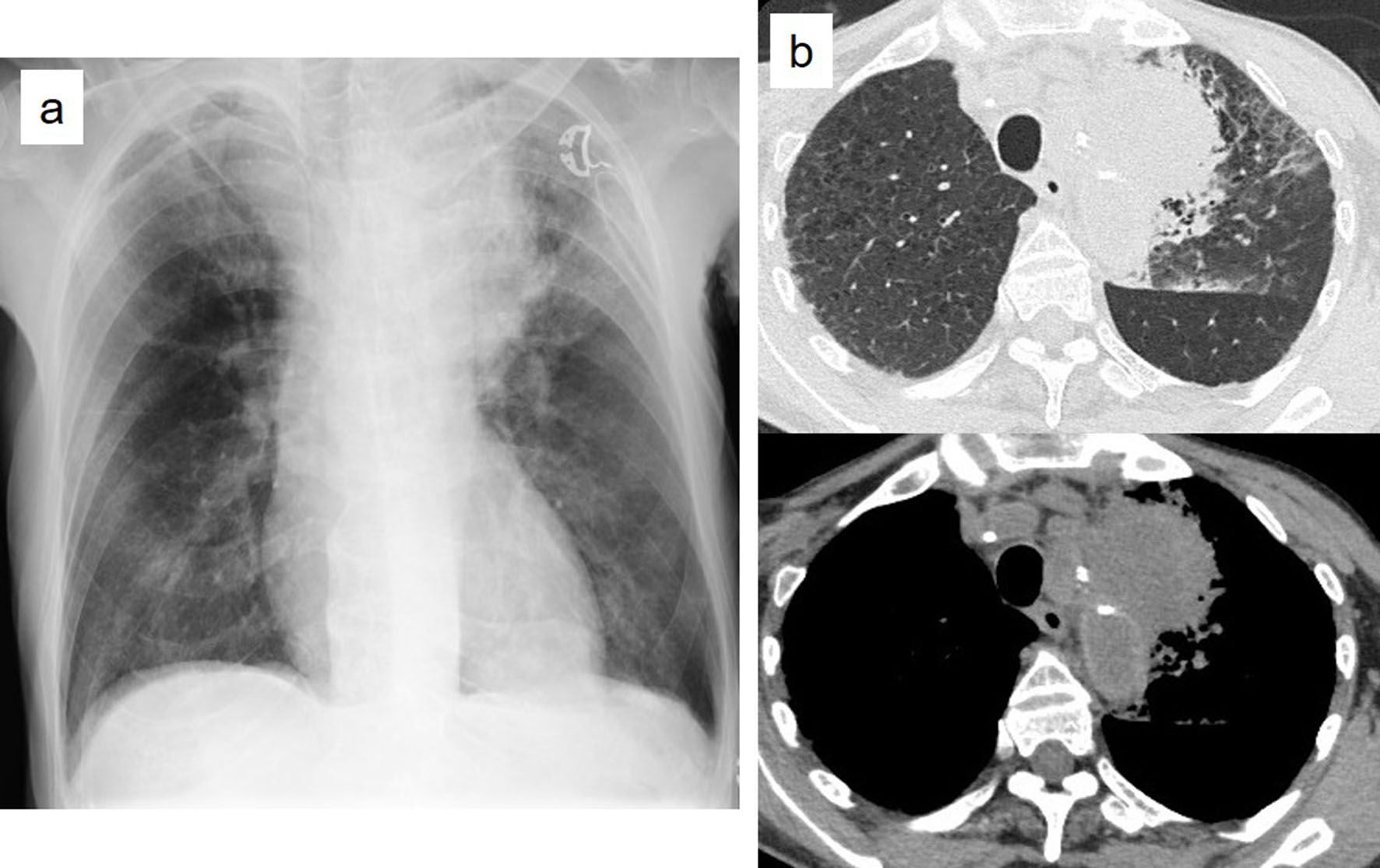
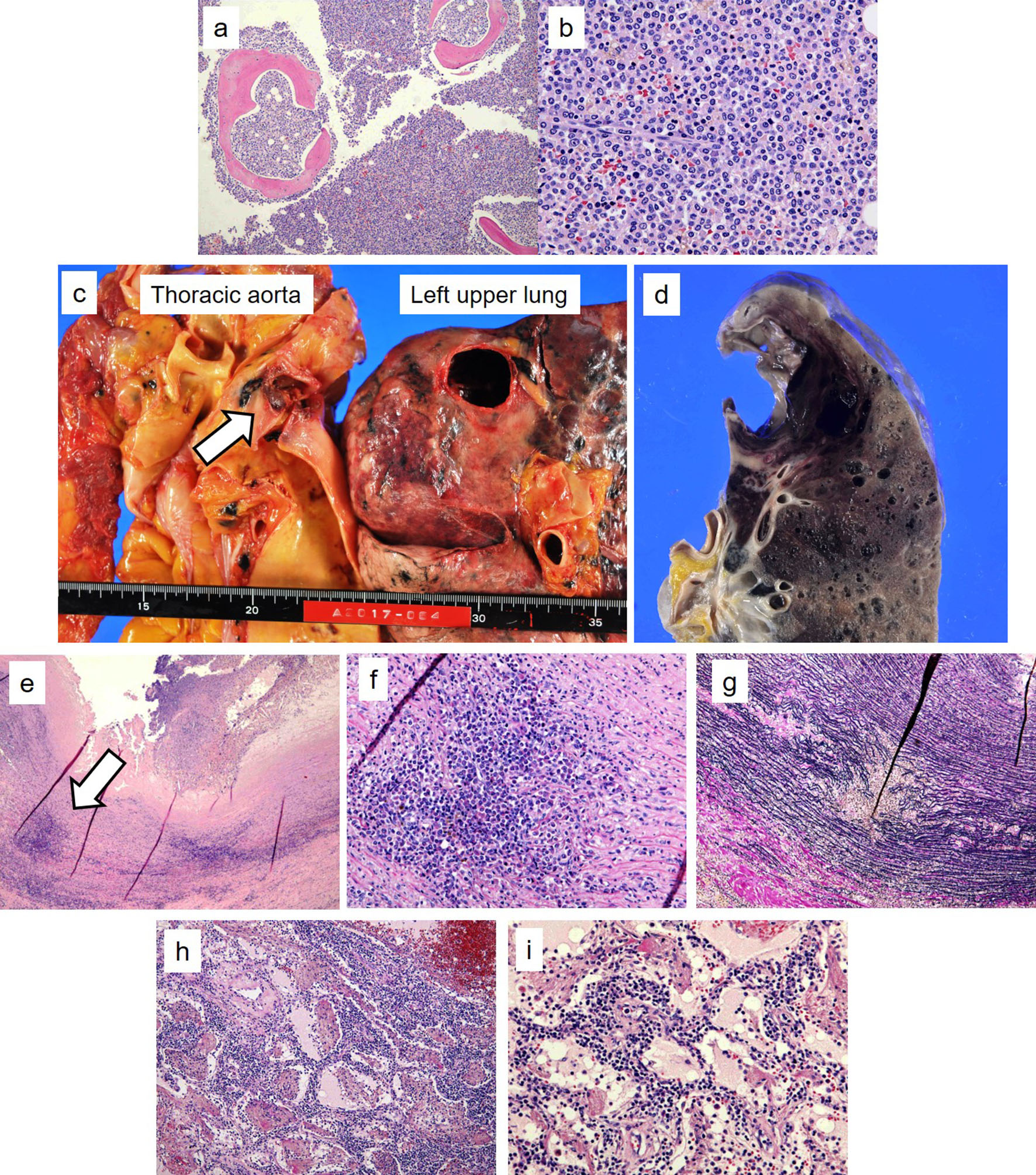
Tables
| Bone marrow examination | |
| NCC | 2,013 × 109/L |
| Mgk | 0.06 × 109/L |
| Basophilic erythroblast | 2.0% |
| Polychromatic erythroblast | 7.2% |
| Orthochromatic erythroblast | 1.4% |
| Myeloblast | 9.0% |
| Promyelocytes | 1.2% |
| Myelocytes | 25.6% |
| Metamyelocytes | 2.8% |
| Stab cells | 3.8% |
| Segmented cells | 0.2% |
| Eosinophil | 0.2% |
| Promonocytes | 3.4% |
| Monocytes | 27.2% |
| Lymphocytes | 2.6% |
| APTT: activated partial thromboplastin; PT: prothrombin time; INR: international normalized ratio; FDP: fibrinogen degradation product; F: coagulation factor. | |
| Complete blood cell count | |
| White blood cell | 866 × 109/L |
| Blast | 3.5% |
| Myelocyte | 18.5% |
| Metamyelocytes | 7.5% |
| Stab cells | 1.5% |
| Segmented cells | 25.0% |
| Lymphocyte | 7.5% |
| Monocyte | 36.5% |
| Hemoglobin | 6.7 g/dL |
| Platelet count | 19 × 109/L |
| Coagulation test | |
| PT | 17.0 s |
| PT-INR | 1.38 |
| APTT | 44.8 s |
| Fibrinogen | 533 mg/dL |
| FDP | 10.8 µg/mL |
| Blood chemistry/serological test | |
| Total protein | 7.1 g/dL |
| Albumin | 2.6 g/dL |
| Aspartate transaminase | 67 U/L |
| Alanine aminotransferase | 39 U/L |
| Lactate dehydrogenase | 929 U/L |
| Alkaline phosphatase | 737 U/L |
| Gamma-glutamyl transpeptidase | 114 U/L |
| Total bilirubin | 0.7 mg/dL |
| Uric acid | 4.6 mg/dL |
| Urea nitrogen | 60 mg/dL |
| Creatinine | 3.00 mg/mL |
| Creatine phosphokinase | 35 U/L |
| C-reactive protein | 16.23 mg/dL |
| Infection-associated test | |
| Blood culture (2 set) | Negative |
| Sputum culture | Resident bacteria of the oral cavity |
| Gaffky scale (3 set) | 0 |