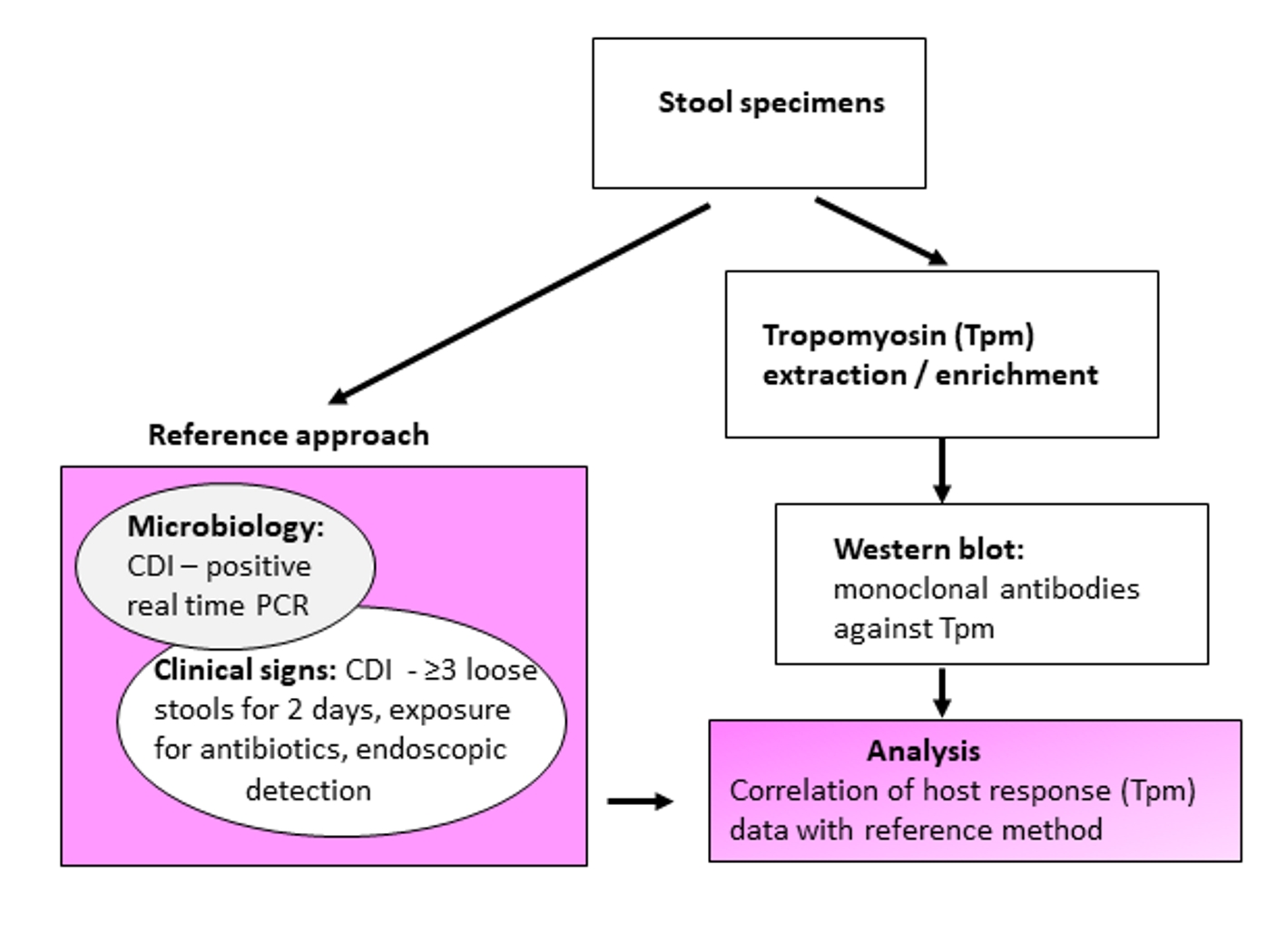
Figure 1. Schematic experimental design and evaluation of tropomyosin (Tpm) as a Clostridium difficile infection (CDI) biomarker.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 2, February 2019, pages 98-105
Cytoskeletal Tropomyosin as a Biomarker in Clostridium difficile Infection
Figures

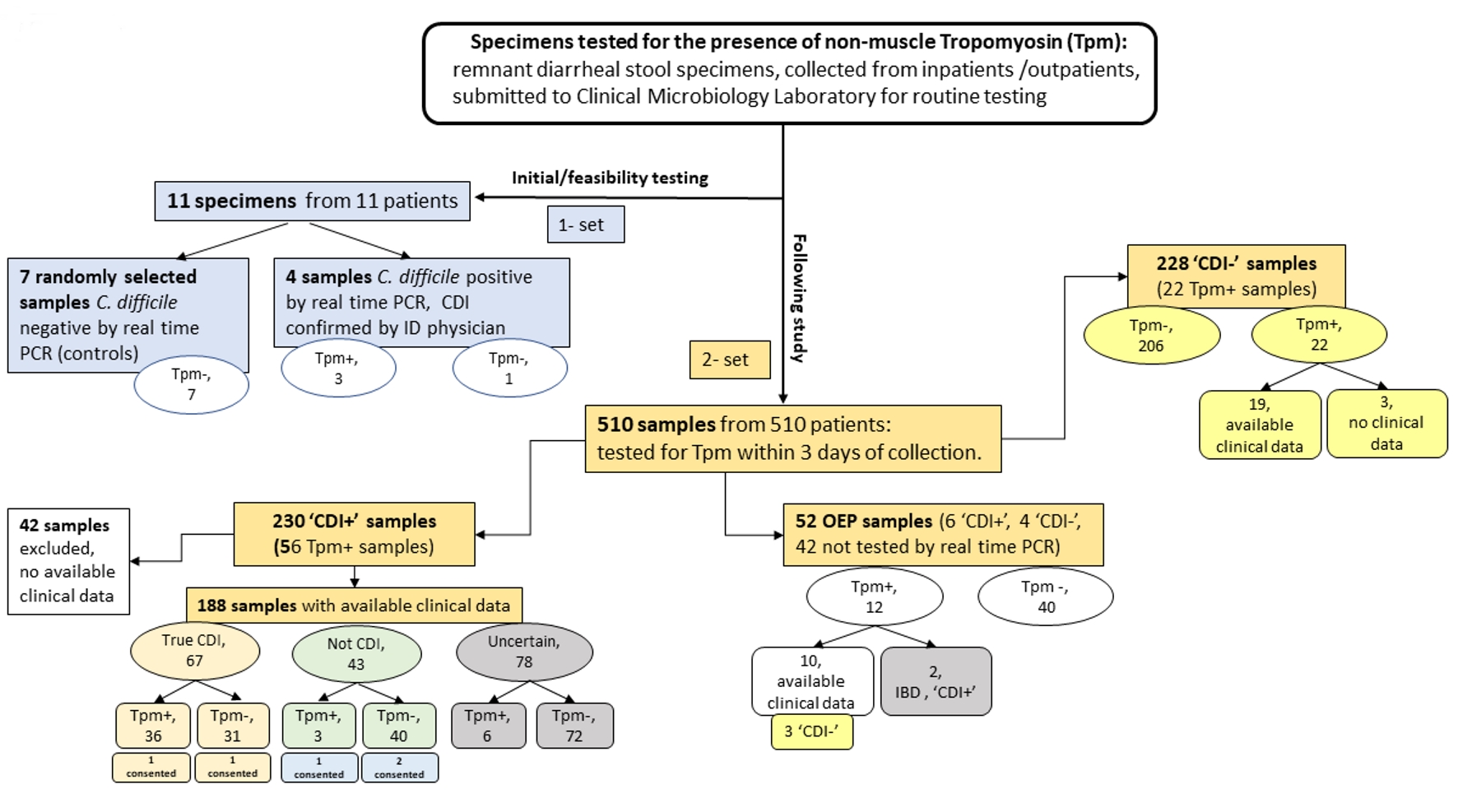
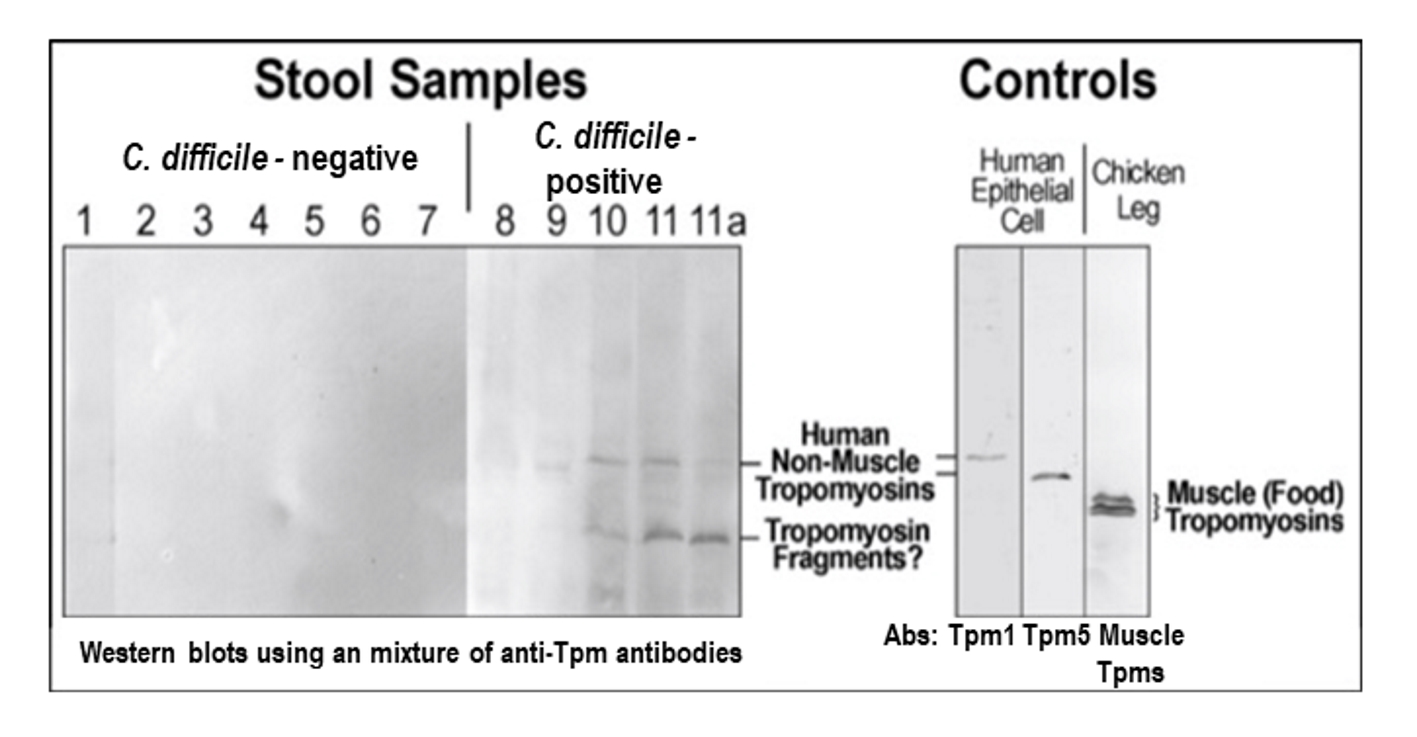
Tables
| True CDI, n | Not CDI, n | Uncertain diagnosis, n | Total | |
|---|---|---|---|---|
| In brackets % of total samples in corresponding categories from total samples in “CDI+” group. Underlined numbers are the most important indications. “true CDI” - “CDI+” subgroup: it denotes specimens collected from patients clinically evaluated as having real infection. “not CDI” - “CDI+” subgroup: it denotes samples collected from patients tested positive by PCR but CDI diagnosis was not confirmed. “Uncertain diagnosis”: specimens tested positive by PCR but the diagnosis is inconclusive. | ||||
| Tpm positive | 36 | 3 | 6 | 45 (24%) |
| Tpm negative | 31 | 40 | 72 | 143 (76%) |
| Total | 67 (35.6%) | 43 (23%) | 78 (41.5%) | 188 |
| True CDI, n | Not CDI, n | Uncertain diagnosis, n | Total | |
|---|---|---|---|---|
| “true CDI” - “CDI+” subgroup: it denotes specimens collected from inpatients clinically evaluated as having real infection. “not CDI” - “CDI+” subgroup: it denotes samples collected from inpatients tested positive by PCR but CDI diagnosis was not confirmed. “Uncertain diagnosis”: specimens tested positive by PCR, but diagnosis is inconclusive. | ||||
| Tpm positive | 9 | 1 | 1 | 11 |
| Tpm negative | 9 | 13 | 16 | 38 |
| Total | 18 | 14 | 17 | 49 |
| Tested samples, n | Tpm positive, n (%, in corresponding category) | Samples with available clinical signs, n | |
|---|---|---|---|
| *: only Tpm-positive samples; CDI+: remnant diarrheal stool specimens submitted to clinical microbiology laboratory that tested positive for C. difficile toxin DNA by PCR method (Xpert® C. difficile/Epi); CDI-: emnant diarrheal stool specimens submitted to clinical microbiology laboratory that tested negative for C. difficile toxin DNA by PCR method (Xpert® C. difficile/Epi); OEP: specimens tested for the presence of other enteric pathogens or parasites by routine testing methods. | |||
| CDI- | 228 | 22 (9.7) | 19* |
| CDI+ | 230 | 56 (24.3) | 188 |
| OEP | 52 | 12 (23) | 10* |
| Total | 510 | 90 (17.7) | 217 |
| Biomarker | Clinical indication/prediction | Role in immunopathogenesis | Specific/sensitive to CDI |
|---|---|---|---|
| *: adapted from reference [10]. Permission was granted. pMK2: pyruvate kinase M2; IL-8: interleukin-8. | |||
| Lactoferrin | Colonic inflammation, CDI severity (when the level is elevated) | The innate inflammatory response; related to level of neutrophils translocation | no/no |
| Calprotectin | Intestinal inflammatory conditions (when the level is elevated); CDI severity | The innate inflammatory response: correlates with level of released neutrophils | no/no |
| IL-8 | CDI severity (when elevated) | Involved in the recruitment of neutrophils to sites of infection | no/yes |
| IL-23 | May relate to CDI recurrence (when the level is decreased) | The lack of a robust immunological response | no/no |
| pMK2 | The presence of toxigenic C. difficile (when the level is elevated) | A key mediator of p38-dependent inflammation | no/- |
| pp38 | Symptomatic CDI in pediatrics (when the level is elevated) | Activation of p38 protein pathway | yes/no |