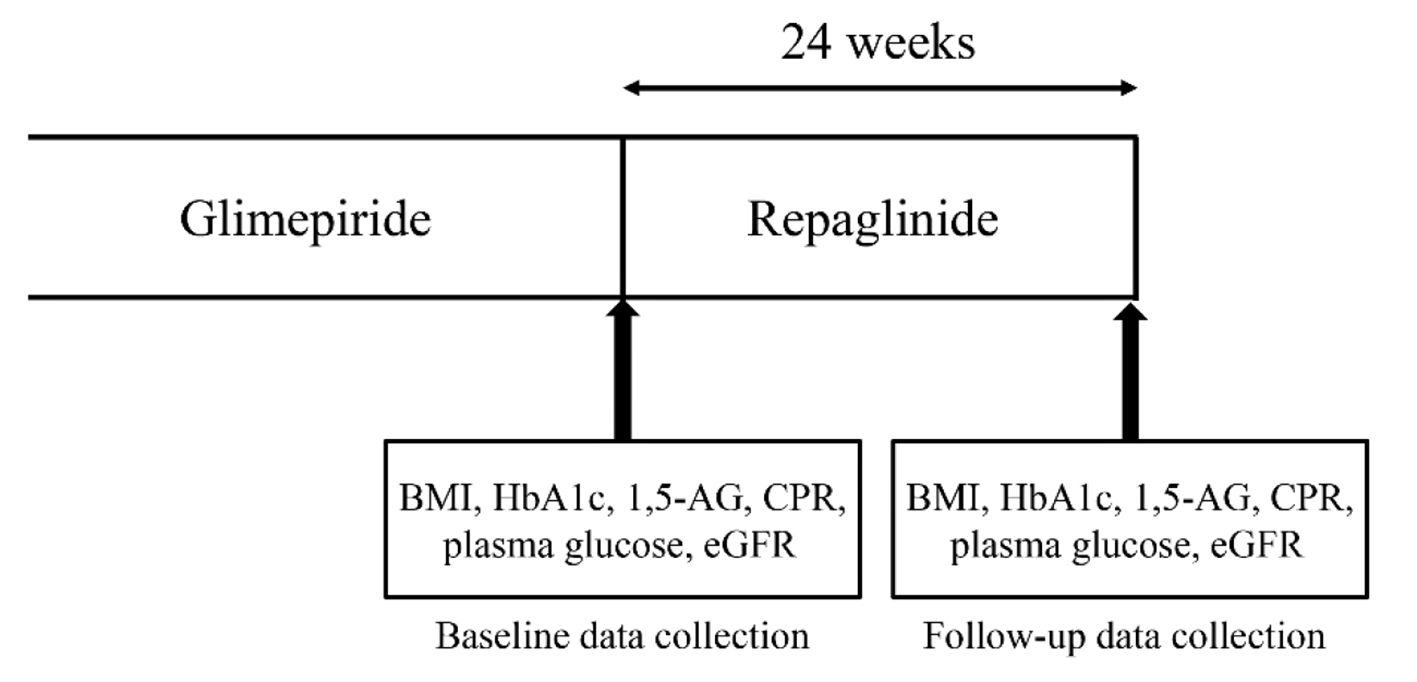
Figure 1. Study protocol. Japanese patients with type 2 diabetes were enrolled and followed-up for 24 weeks after switching from glimepiride to repaglinide. Clinical metabolic parameters were measured and recorded at weeks 0 (baseline) and 24.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 11, November 2018, pages 838-842
Effects of Repaglinide Versus Glimepiride on 1,5-Anhydroglucutol and Glycated Hemoglobin Levels in Japanese Patients With Type 2 Diabetes
Figures

Table
| Data are expressed as mean ± standard deviation. BMI: body mass index; HbA1c: glycated hemoglobin; CPR: C-peptide immunoreactivity; 1,5-AG: 1,5-anhydroglucitol; eGFR: estimated glomerular filtration rate. | |
| Age (years) | 68.3 ± 8.10 |
| Sex (male/female) | 7/3 |
| Body weight (kg) | 59.5 ± 12.3 |
| BMI (kg/m2) | 23.9 ± 5.12 |
| Duration of diabetes (years) | 5.5 (4 - 7.8) |
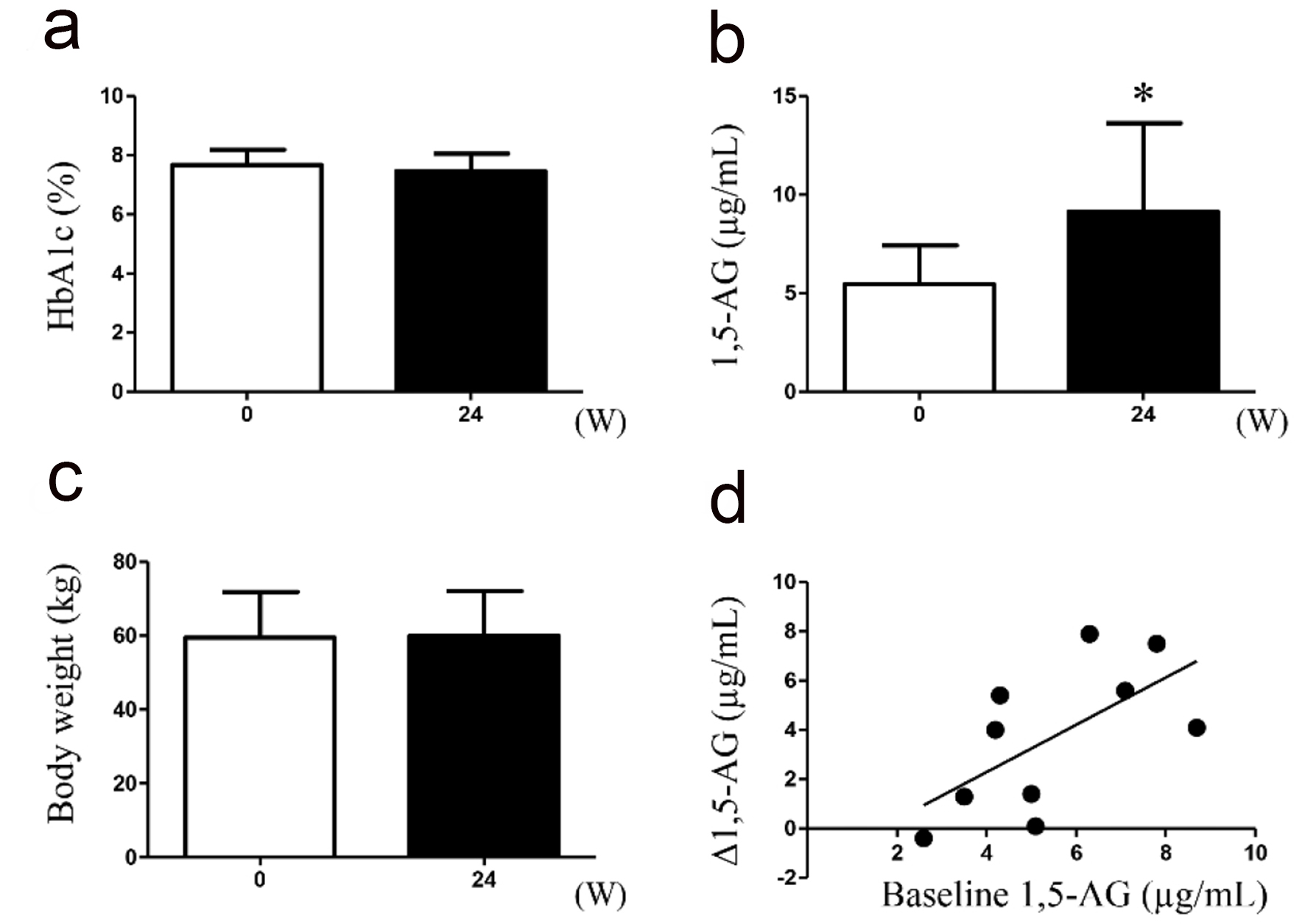
| HbA1c (%) | 7.7 ± 0.52 |
| Random plasma glucose (mg/dL) | 152 ± 51 |
| Random serum CPR (ng/mL) | 2.6 (1.6 - 3.0) |
| Baseline 1,5-AG (µg/mL) | 5.5 ± 2.0 |
| eGFR (mL/min/1.73 m2) | 73 ± 10 |
| Dyslipidemia (n) | 4 |
| Hypertension (n) | 3 |
| Anti-hyperglycemic drugs | |
| Metformin (n) | 8 |
| Alpha-glucosidase inhibitor (n) | 1 |
| Pioglitazone (n) | 5 |
| Dipeptidyl peptidase-4 inhibitor (n) | 4 |