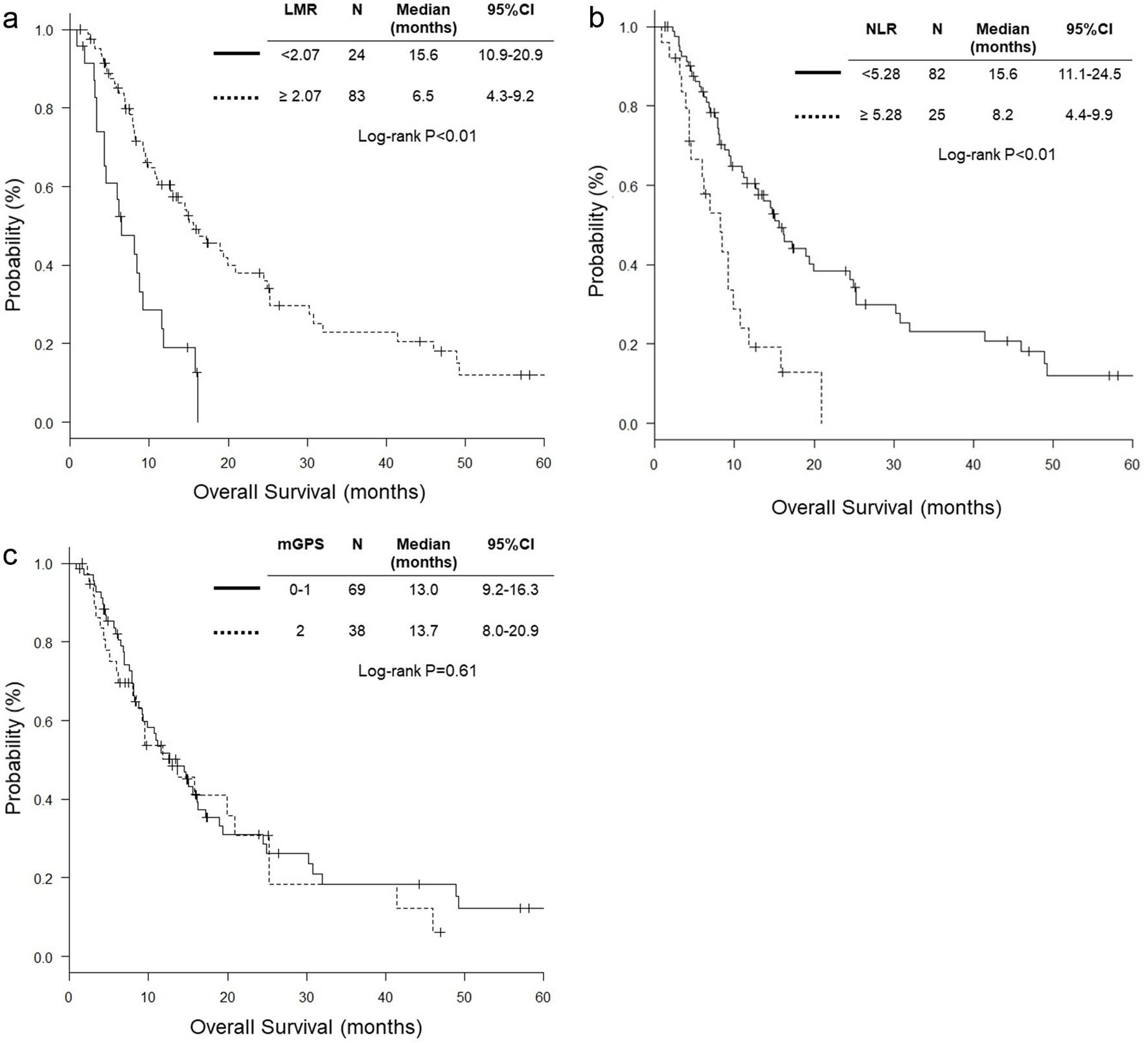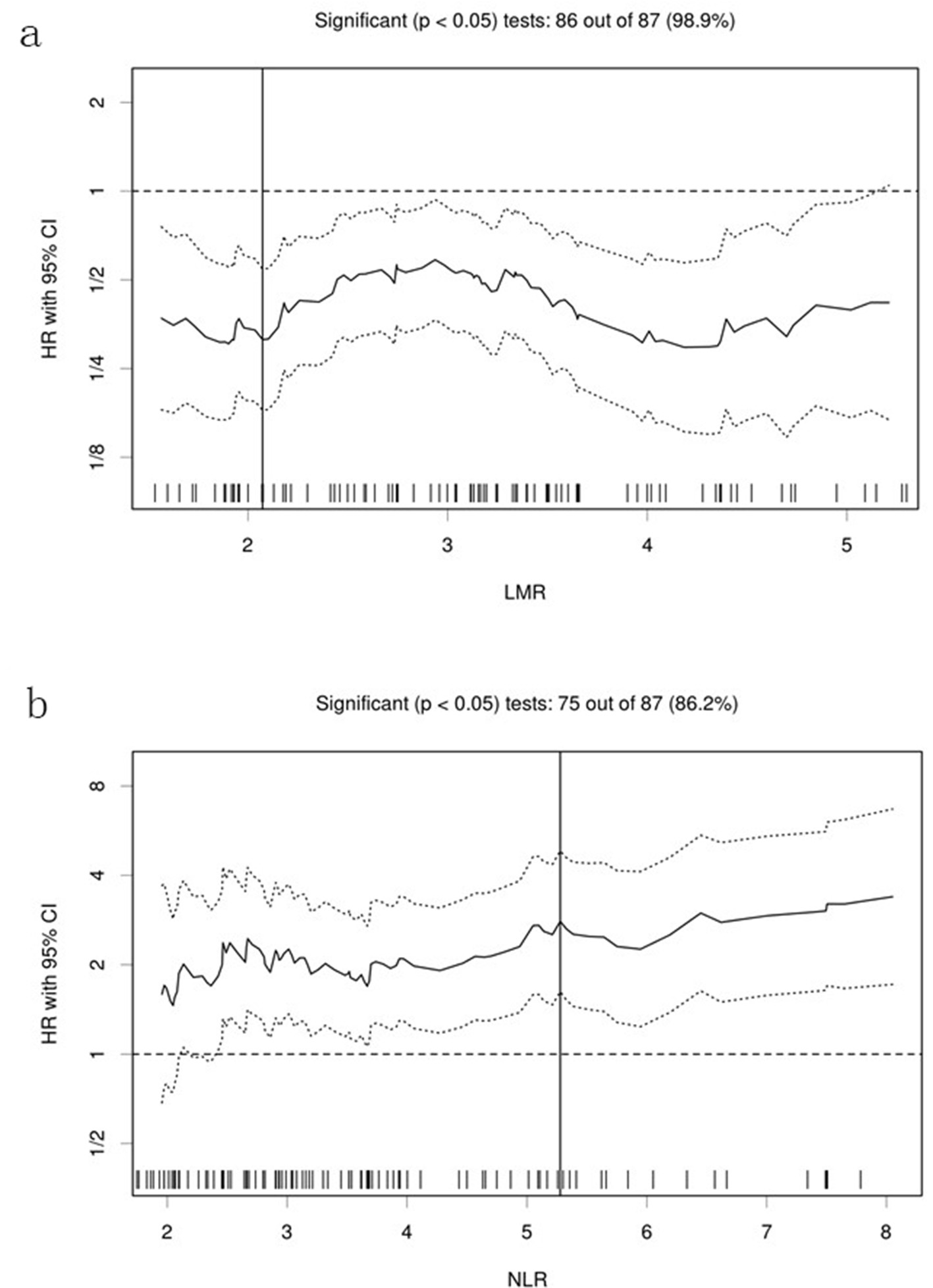
Figure 1. Hazard ratios and cutoff points of LMR and NLR for overall survival of patients with pulmonary squamous cell carcinoma. (a) LMR; (b) NLR. The plots were determined using Cutoff Finder. The vertical lines are the optimal cutoff points providing the most significant split (log-rank test). LMR: lymphocyte to monocyte ratio; NLR: neutrophil to lymphocyte ratio; HR: hazard ratio; OS: overall survival.