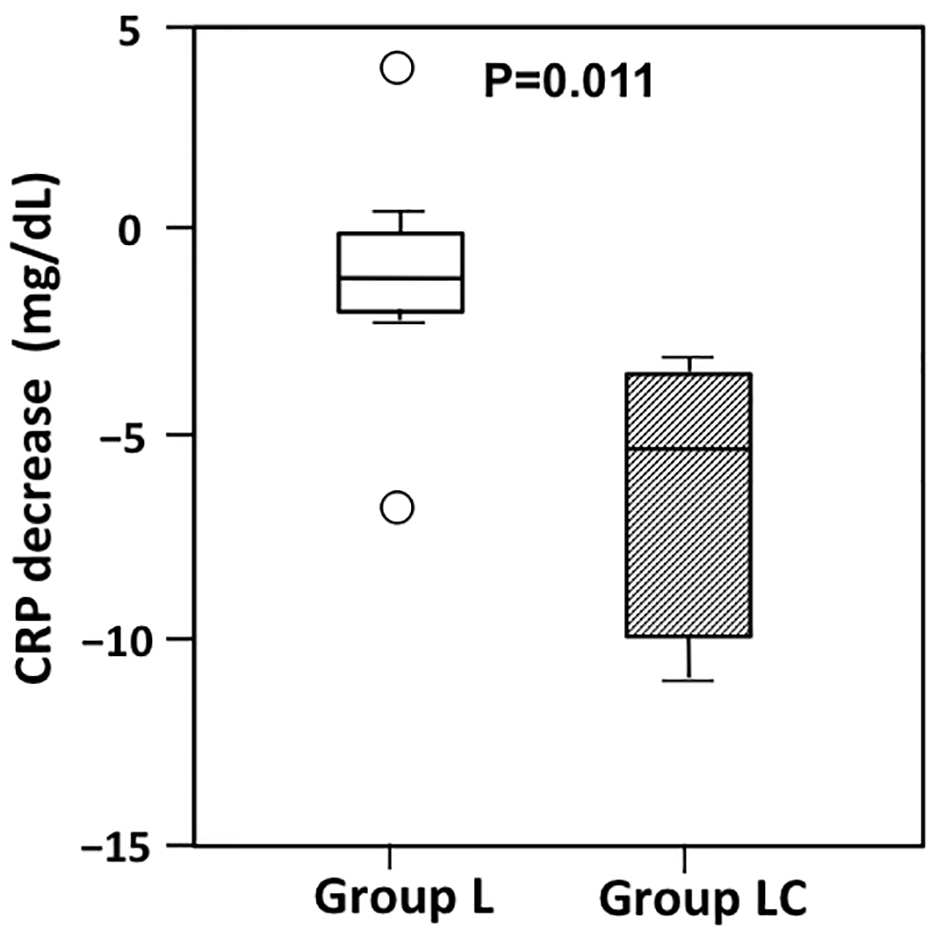
Figure 1. Changes in C-reactive protein (CRP) from postoperative day (POD) 3 to POD7 in patients receiving peripheral parenteral nutrition (PPN) alone (group L) or intravenous carnitine in addition to PPN (group LC). The decrease in CRP from POD3 to POD7 was significantly greater in group LC than in Group L (P = 0.011). The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. Circles show outliers.