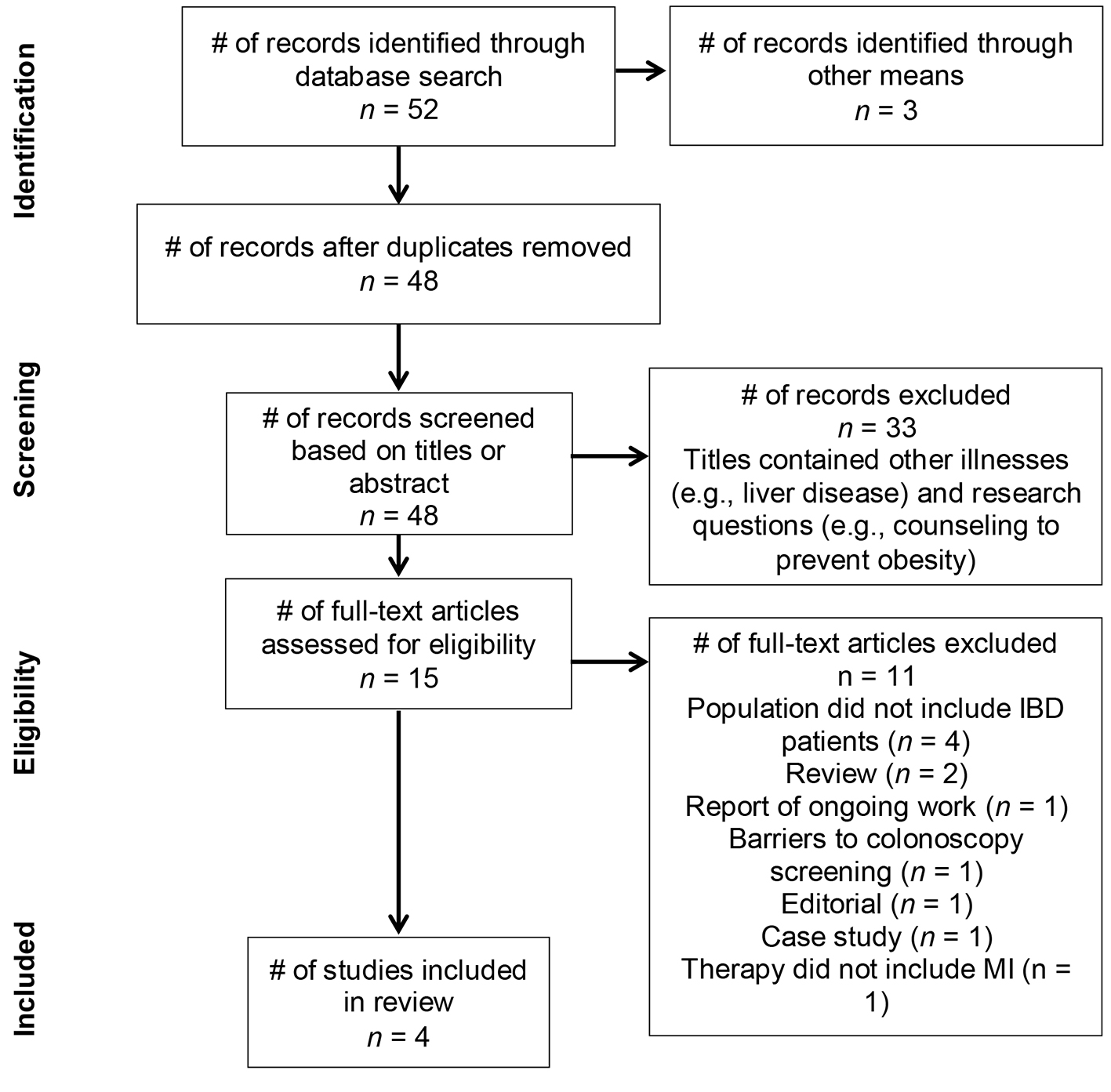
Figure 1. PRISMA flow diagram detailing trial search and review tiers.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 9, Number 8, August 2017, pages 659-666
The Influence of Motivational Interviewing on Patients With Inflammatory Bowel Disease: A Systematic Review of the Literature
Figure

Tables
| Article | Method | Participants | Intervention type and duration | Outcomes measured | Measuring instrument | Outcomes results |
|---|---|---|---|---|---|---|
| Mocciaro et al, 2014 [21] | Quasi-experimental | 45 adult patients with IBD | Gastroenterologists engaged in motivational interviewing during one 45-min routine consultation with patients | Patient-reported satisfaction with provider, perceived provider empathy, provider communication skills, attendance, smoking cessation, adherence | Patient self-report and presence at follow-up visit | Patient-reported satisfaction with provider, perceived provider empathy, and provider communication skills were significantly higher than patient ratings for prior experiences with providers. Patients had 100% attendance for the follow-up appointment; 60% of patients quit smoking from initial meeting to follow-up; patients had 95.6% adherence at follow-up. |
| Berrill et al, 2014 [23] | Randomized controlled trial | 66 adult patients with IBD | A six-session multi-convergent intervention occurring over 16 weeks. The first session involved a 40-min motivational interviewing session. | Quality of life, advice-seeking behavior | Patient self-report | Intervention group did not experience significantly greater quality of life. Subgroup of intervention group experiencing IBS-symptoms experienced a significant increase in quality of life at conclusion of study. Intervention group was significantly more likely to seek advice at 12 month follow-up. |
| Moshkovska et al, 2011 [22] | Randomized controlled trial | 71 adult patients with ulcerative colitis | A medical doctor conducted a 20- to 30-min one-on-one session with each patient that helped them identify barriers to adherence. Patients were also given education and motivational interviewing to attend to concerns. | Adherence | High performance liquid chromatography to assess urine sample | At 48-week follow-up, adherence was significantly greater for intervention group, which indicated a smaller decline in adherence since both groups experienced a reduction in adherence rates over the course of the study. |
| Cook et al, 2010 [24] | Quasi-experimental | 278 adult patients with ulcerative colitis | Registered nurses conducted telephone sessions with cognitive behavioral and motivational interviewing techniques. Over 6-month intervention, patients received an average of four calls lasting 13 min. | Adherence (defined as months of completed treatment) | Patient self-report during phone calls | Over 6 months, patients had higher rates of self-reported adherence than a comparison population baseline. |
| Source | Sequence generation | Allocation concealment | Blinding (patient-reported outcomes) | Incomplete outcome data addressed? | Free of selective reporting? | Free of other bias? | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Judgment | Description | Judgment | Description | Judgment | Description | Judgment | Description | Judgment | Description | Judgment | Description | |
| Berrill et al [23] | Yes | Comment: Patients were randomized to an active or control group using a blocked randomization method | Yes | Comment: Sequences for intervention were placed in sealed opaque envelopes | No | Comment: Authors state participants were not blinded | Yes | Comment: 8 patients did not attend intervention; 6 dropped out during study. In control group, 1 patient was lost to follow-up | Yes | Comment: Methods and outcomes all reported | Yes | Comment: No other biases noted in this study |
| Cook et al [24] | No | Comment: There was no control group | No | Comment: Nurses delivering intervention were aware of patient status in group | No | Comment: Participants were contacted by study personnel who explained program. Unclear what exactly was explained | Yes | Comment: Authors reported number of patients who dropped out and compared their demographics and baseline adherence to patients’ who remained in study. | Yes | Comment: Methods and outcomes all reported | Yes | Comment: No other biases noted in this study |
| Mocciaro et al [21] | No | Comment: There was no control group | No | Comment: Physicians delivering intervention were aware of patient status in group | Unclear | Comment: Not specifically stated if patients knew details of intervention | Yes | Comment: Authors stated all patients attended follow-up visit | Yes | Comment: Methods and outcomes all reported | Yes | Comment: No other biases noted in this study |
| Moshkovska et al [22] | Yes | Comment: Computer-generated randomization schedule was used. Blocking was also used. | Yes | Comment: Authors stated that sequentially numbered, opaque, sealed envelopes containing the sequence were used. | Yes | Comment: Authors stated investigator recruiting patients was blinded to content of envelopes, but interventionist and patients were not blinded. | Yes | Comment: Authors reported number of patients who withdrew due to medical reasons and who were lost to follow-up. Authors stated withdrawal rates were similar for intervention and control groups. | Yes | Comment: Methods and outcomes all reported | Yes | Comment: No other biases noted in this study |