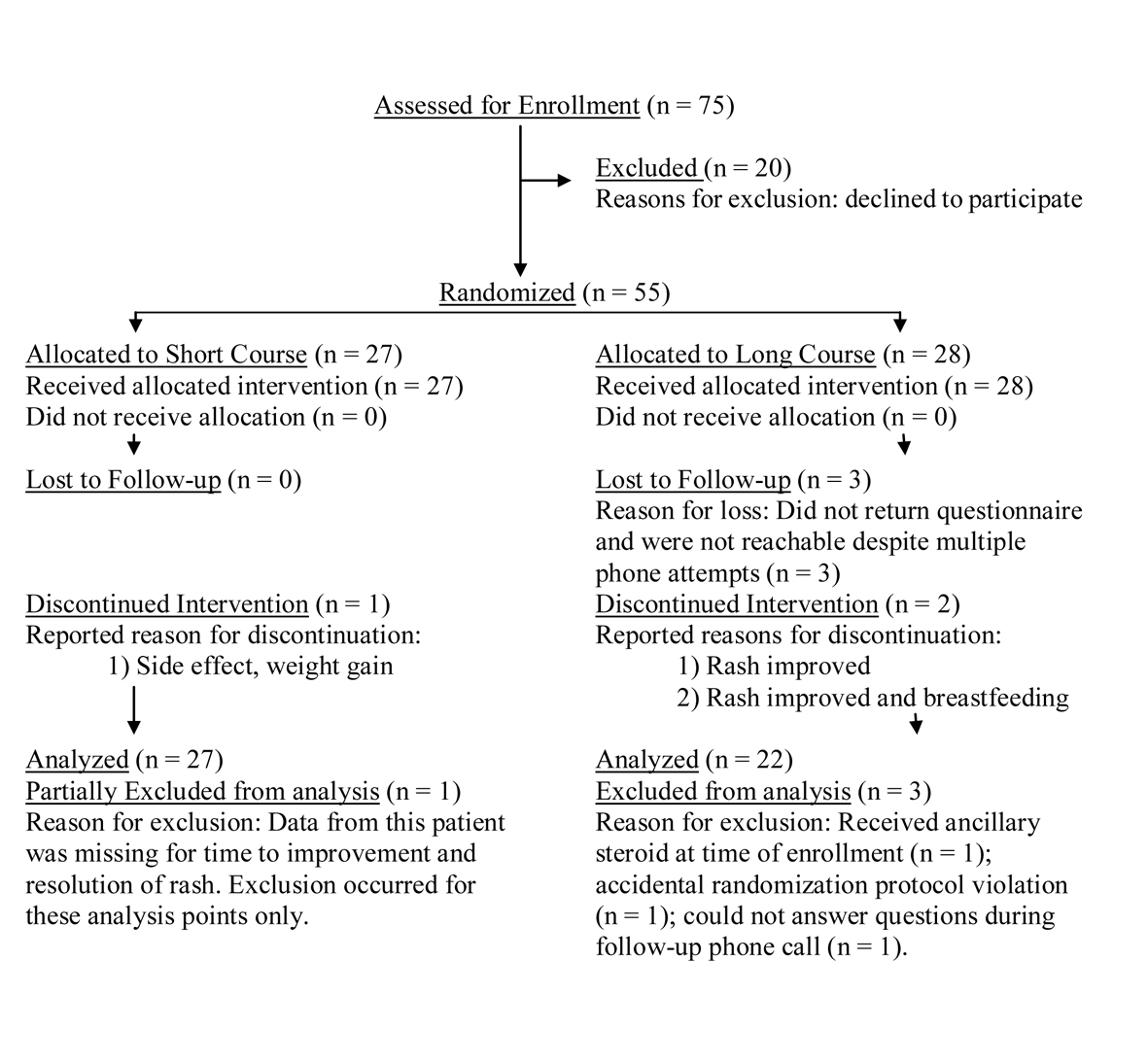
Figure 1. Patients enrollment flowchart.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 6, Number 6, December 2014, pages 429-434
Treatment of Severe Poison Ivy: A Randomized, Controlled Trial of Long Versus Short Course Oral Prednisone
Figure

Tables
| Short course | Long course | Total | |
|---|---|---|---|
| Male | 7 | 6 | 13 |
| Female | 20 | 16 | 36 |
| Age mean | 40.89 | 34.09 | 37.88 (SD 17.45, range 15 - 86) |
| Enrollment | |||
| April | 1 | 3 | 4 |
| May | 3 | 3 | 6 |
| June | 9 | 5 | 14 |
| July | 4 | 3 | 7 |
| August | 7 | 2 | 9 |
| September | 3 | 6 | 9 |
| Short course/27 | Long course/22 | Total/49 | P value | Mean difference | |
|---|---|---|---|---|---|
| Questionnaire | 18 | 15 | 33 | ||
| Telephone | 9 | 7 | 18 | ||
| Noncompliance reported | 1 | 2 | 3 | 0.581 | |
| Reported improvement of rash | 22 | 21 | 43 | 0.204 | |
| Mean time to improvement | 4.42 days (SD 4.13 days) | 2.93 days (SD 1.23 days) | 0.109 | -1.49 days | |
| Mean time to resolution | 14.63 days (SD 8.87 days) | 11.7 days (SD 7.39 days) | 0.23 | -2.93 days | |
| Rash return?/td> | 4 | 3 | 7 | 0.91 | |
| Return in same location?/td> | 4 | 2 | 6 | ||
| Side effects | 3 | 0 | 3 | 0.239 | |
| Use of other medication: | 15 | 5 | 20 | 0.02 |
| Short course/27 | Long course/22 | Total/49 | P value | 95% confidence intervals | |
|---|---|---|---|---|---|
| Use of other medication: | 15 | 5 | 20 | 0.02 | |
| Prednisone, Rx | 9 | 2 | 11 | ||
| Depo Medrol, Rx | 1 | 0 | 1 | ||
| Triamcinolone, Rx | 1 | 0 | 1 | ||
| Calamine, OTC | 2 | 4 | 6 | ||
| Antihistamine, OTC | 2 | 2 | 4 | ||
| Hydrocortisone cream, OTC | 3 | 0 | 3 | ||
| Gold bond lotion, OTC | 1 | 0 | 1 | ||
| Other lotion, OTC | 2 | 0 | 2 | ||
| Event rate | 22.70% | 55.60% | |||
| Absolute risk reduction | 32.90% | 0.139 - 0.385 | |||
| Relative risk reduction | 59.20% | 0.331 - 0.611 | |||
| Number needed to treat | 3.05 | 2.60 - 7.19 | |||
| Relative risk | 0.41 | 0.18 - 0.95 | |||
| Odds ratio | 0.24 | 0.067 - 0.824 |