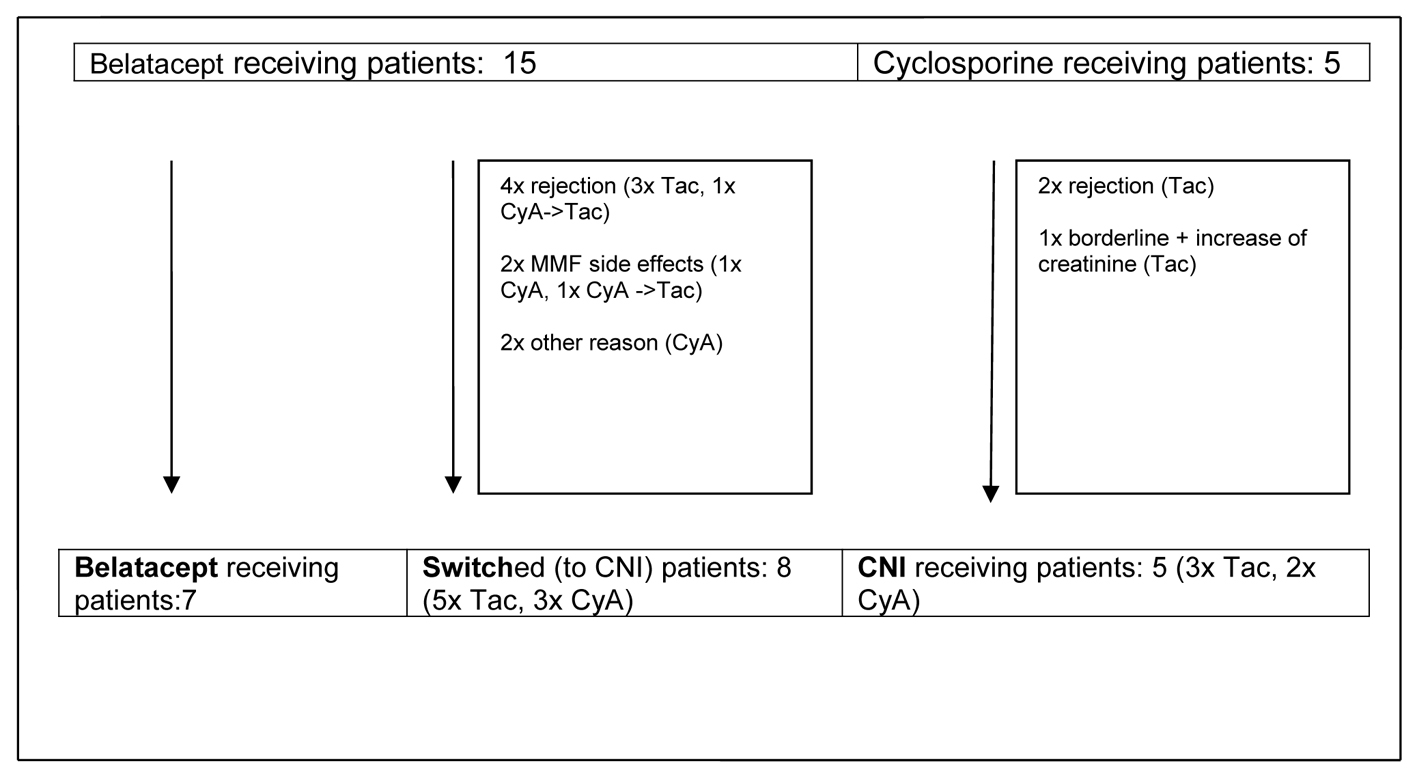
Figure 1. Development of observational groups up to month 14 post-transplant. CyA: cyclosporine A; Tac: tacrolimus; CNI: calcineurin inhibitor; MMF: mycophenolate mofetil.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 6, Number 2, April 2014, pages 98-110
Ten Years Experience With Belatacept-Based Immunosuppression After Kidney Transplantation
Figures

Tables
| Pat. No. | Age (years) | Gender | BMI (kg/m2) | No. of CRF | Tye of organ | Donor age (years) | CIT (min) | MM | Underlaying disease |
|---|---|---|---|---|---|---|---|---|---|
| BMI: body mass index; CRF: cardiovascular risk factors; CIT: cold ischemia time; MM: mismatch. *: switched patients. | |||||||||
| Patients initially receiving belatacept (*switched patients) | |||||||||
| 1* | 53 | M | 29.8 | 3 | LRD | 57 | 222 | 2 | Nephrosklerosis |
| 2 | 44 | M | 19.4 | 3 | DOD | 23 | 844 | 2 | Unknown |
| 3* | 46 | M | 23.6 | 1 | LNRD | 45 | 226 | 5 | Chronic glomerulonephritis |
| 4* | 32 | M | 23.1 | 1 | DOD | 58 | 980 | 0 | IgA nephropathy |
| 5* | 41 | M | 21 | 2 | DOD | 46 | 1,273 | 1 | IgA nephropathy |
| 6 | 53 | M | 25.1 | 3 | DOD | 38 | 849 | 2 | Polycystic kidney disease |
| 7 | 40 | M | 30 | 2 | LRD | 64 | 205 | 3 | IgA nephropathy |
| 8 | 27 | M | 18.9 | 1 | DOD | 37 | 717 | 2 | Obstructive nephropathy |
| 9* | 45 | M | 27.5 | 2 | DOD | 29 | 1,430 | 2 | Nephrosklerosis |
| 10 | 41 | M | 19.5 | 2 | DOD | 58 | 821 | 4 | Unknown |
| 11* | 25 | M | 25.6 | 2 | DOD | 58 | 559 | 0 | IgA nephropathy |
| 12* | 70 | M | 24.2 | 2 | DOD | 36 | 820 | 1 | Nephrosklerosis |
| 13 | 32 | M | 22.3 | 3 | DOD | 53 | 1,233 | 3 | Obstructive nephropathy |
| 14* | 42 | F | 20.7 | 2 | DOD | 58 | 856 | 0 | Post-streptococcal glomerulonephritis |
| 15 | 32 | F | 18.1 | 2 | DOD | 54 | 786 | 0 | Hereditary nephritis |
| Patients initially receiving CyA | |||||||||
| 16 | 60 | M | 22.4 | 2 | DOD | 17 | 841 | 1 | Chronic pyelonephritis |
| 17 | 36 | M | 22.7 | 1 | DOD | 49 | 784 | 2 | Chronic glomerulonephritis |
| 18 | 24 | M | 20.2 | 1 | DOD | 48 | 1,179 | 3 | Cystinosis |
| 19 | 41 | M | 23.5 | 2 | DOD | 58 | 1,118 | 0 | IgA nephropathy |
| 20 | 58 | F | 27.8 | 1 | DOD | 38 | 1,154 | 2 | Chronic pyelonephritis |
| Pat. No. | CMV donor | CMV recipient | Viral infection | Bacterial infection | malignancies |
|---|---|---|---|---|---|
| CMV: cytomegalovirus; CIN: cervical intraepithelial neoplasia; CNI: calcineurin inhibitor. | |||||
| Patients initial treated with belatacept | |||||
| 2 | pos | pos | - | - | - |
| 6 | neg | pos | - | - | 2× basalioma (year 5) |
| Bronchial carcinoma (year 8) | |||||
| 7 | pos | neg | CMV (month 5) | - | - |
| 8 | neg | neg | - | - | - |
| 10 | neg | pos | Herpes zoster (month 2) | - | - |
| 13 | neg | pos | - | Endocarditis, pleural empyema | - |
| 15 | pos | neg | CMV (month 8) | Urosepsis (month 6) | CIN cervix (year 7) |
| Patients switched from belatacept to CNI | |||||
| 1 | pos | neg | 3× CMV (month 5, 6, 7) | - | - |
| 3 | pos | neg | Herpes zoster (year 2) | - | - |
| 4 | neg | neg | - | - | - |
| 5 | neg | pos | - | - | - |
| 9 | neg | pos | - | - | - |
| 11 | neg | pos | - | - | - |
| 12 | pos | pos | CMV (month 1, 3) | Urosepsis (month 3) | Bronchial carcinoma (year 2) |
| 14 | pos | pos | - | - | - |
| CNI-treated patients | |||||
| 16 | pos | neg | - | - | Basalioma (year 8) |
| 17 | pos | neg | CMV (month 6) | Urosepsis (year 6)- | - |
| 18 | pos | neg | CMV (month 2) | - | - |
| 19 | pos | neg | CMV (month 4) | - | 2× basalioma (year 6, 9) |
| 20 | pos | pos | - | Pseudomembraneous colitis (month 2) | - |
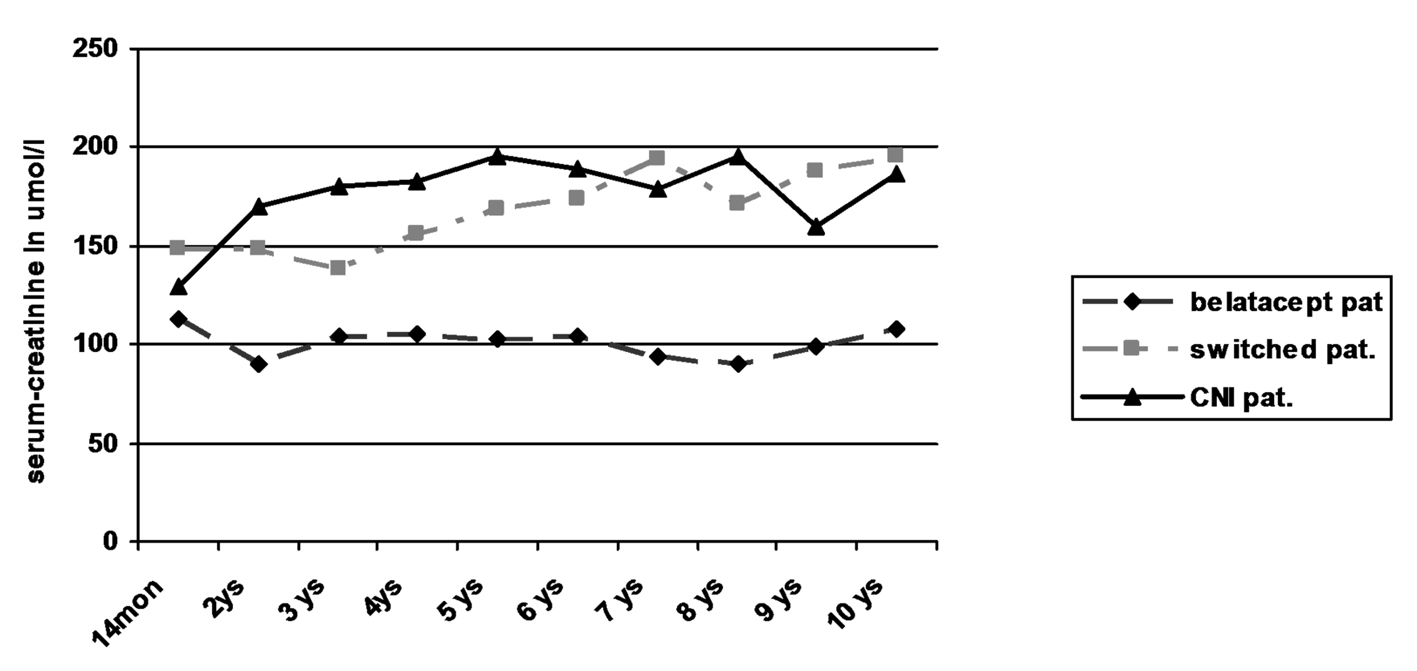
| Pat. No. | 14 | 2 yrs | 3 yrs | 4 yrs | 5 yrs | 6 yrs | 7 yrs | 8 yrs | 9 yrs | 10 yrs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mon | |||||||||||
| Bela: belatacept; TAC: tacrolimus; CyA: ciclosporine A; Aza: azathioprine. | |||||||||||
| CNI-free patients treated with belatacept (1) | |||||||||||
| 2 | Immunosup | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela |
| Creatinine, μmol/L | 70 | 71 | 73 | 66 | 77 | 72 | 66 | 68 | 76 | 76 | |
| 6 | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Patient died | ||
| 86 | 86 | 96 | 91 | - | 82 | - | 75 | ||||
| 7 | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | |
| 146 | 143 | 151 | 132 | - | 139 | - | - | - | 128 | ||
| 8 | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | |
| 117 | 101 | 113 | 107 | 94 | 109 | 95 | 90 | 98 | 97 | ||
| 10 | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | |
| 113 | 112 | 116 | 116 | 134 | 118 | 108 | 108 | 115 | 118 | ||
| 13 | Bela | Patient died | |||||||||
| 156 | |||||||||||
| 15 | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | Bela | |
| 76 | 93 | 89 | 103 | 112 | 98 | 92 | 98 | 99 | 91 | ||
| Median | 113 | 103 | 105 | 105 | 103 | 104 | 94 | 90 | 99 | 97 | |
| Patients switched from belatacept to CNI (2) | |||||||||||
| 1 | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | |
| 134 | 149 | 149 | 131 | 149 | 165 | 182 | 147 | 181 | 194 | ||
| 3 | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | |
| 237 | 251 | 250 | 202 | 219 | 182 | 214 | 196 | 194 | 219 | ||
| 4 | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | |
| 189 | 184 | 220 | 180 | 189 | 207 | 206 | 199 | 211 | 197 | ||
| 5 | CyA | CyA | CyA | CyA | CyA | CyA | CyA | CyA | CyA | CyA | |
| 122 | 118 | 126 | 121 | 127 | 116 | 117 | 107 | 102 | 116 | ||
| 9 | Bela | Switch month 14 to CyA; patient died at month 20 | |||||||||
| 115 | |||||||||||
| 11 | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | Tx failure | |
| 149 | 149 | 127 | 182 | 190 | 192 | 262 | 379 | 674 | |||
| 12 | CyA | CyA | Patient died | ||||||||
| 180 | 161 | ||||||||||
| 14 | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | |
| 118 | 130 | 119 | 130 | 110 | 160 | 129 | 125 | 143 | 123 | ||
| Median | 142 | 149 | 138 | 156 | 169 | 174 | 194 | 172 | 188 | 194 | |
| CNI-treated patients (3) | |||||||||||
| 16 | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | Patient died | ||
| 201 | 250 | 219 | 273 | 266 | 274 | 313 | 362 | ||||
| 17 | CyA | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | TAC | |
| 200 | 207 | 182 | 199 | 195 | 189 | 179 | 196 | 207 | 194 | ||
| 18 | TAC | TAC | TAC | TAC | TAC | +Aza | +Aza | +Aza | +Aza | +Aza | |
| 130 | 170 | 180 | 183 | 237 | 266 | 248 | 189 | 179 | 179 | ||
| 19 | CyA | CyA | CyA | CyA | CyA | CyA | CyA | CyA | CyA | CyA | |
| 95 | 138 | 127 | 129 | 113 | 124 | 108 | 122 | 113 | 120 | ||
| 20 | CyA | CyA | CyA | CyA | CyA | +Aza | +Aza | +Aza | +Aza | +Aza | |
| 95 | 107 | 104 | 106 | 126 | 118 | 106 | 102 | 102 | 109 | ||
| Median | 130 | 170 | 180 | 183 | 195 | 189 | 179 | 189 | 146 | 150 | |
| Mann-Whitney U test; P | 1 vs. 2 | 0.04 | 0.005 | 0.026 | 0.015 | 0.067 | 0.009 | 0.009 | 0.009 | 0.019 | 0.056 |
| 1 vs. 3 | 0.268 | 0.03 | 0.052 | 0.052 | 0.063 | 0.017 | 0.032 | 0.016 | 0.114 | 0.111 | |
| 1 vs. 2+3 | 0.046 | 0.002 | 0.01 | 0.007 | 0.026 | 0.002 | 0.003 | 0.002 | 0.014 | 0.029 | |
| Pat. No. | Group | Cardiovascular event |
|---|---|---|
| 13 | Belatacept | Mitral and aortal valve insufficiency (month 4 and 12) |
| 1 | Switch | Abdominal aortic aneurism (year 3); stroke (arteria cerebri media; year 8) |
| 5 | Switch | Stenosis of right external iliac artery (month 11); |
| Stenosis of left and right common iliac artery (year 4) | ||
| 9 | Switch | Sudden heart death (month 20) |
| 11 | Switch | Stenosis of right coronary artery (year 7) |
| 14 | Switch | Stenosis of transplant artery (month 7) |
| 16 | CNI | Aortic valve stenosis with decompensation (year 9) |
| 18 | CNI | Stroke (arteria cerebri media; year 4) |
| Parameter | 14 mon | 3 yrs | 5 yrs | 8 yrs | 10 yrs |
|---|---|---|---|---|---|
| BP: blood pressure; mon: months. | |||||
| Systolic BP median | |||||
| Belatacept | 120 | 130 | 132 | 122 | 120 |
| Switch | 126 | 123 | 130 | 125 | 120 |
| CNI | 135 | 126 | 128 | 139 | 122 |
| Diastolic BP (mmHg; median) | |||||
| Belatacept | 74 | 75 | 77 | 75 | 68 |
| Switch | 79 | 80 | 80 | 79 | 80 |
| CNI | 82 | 76 | 85 | 83 | 74 |
| No. of BPM (median; (range)) | |||||
| Belatacept | 3 (2-5) | 3 (2-5) | 3 (1-5) | 2 (1-5) | 2 (1-4) |
| Switch | 3 (2-4) | 3 (2-6) | 4 (2-6) | 4 (2-4) | 4 (2-4) |
| CNI | 4 (2-4) | 3 (3-4) | 3 (2-4) | 3 (3-4) | 4 (3-5) |
| Cholesterol (mg/dL; median)/triglycerides | |||||
| Belatacept | 193/171 | 233/165 | 186/112 | 226/143 | 232/118 |
| Switch | 174/343 | 195/508 | 162/259 | 176/238 | 197/334 |
| CNI | 209/175 | 186/187 | 205/188 | 255/145 | 231/166 |
| Pat. with lipid lowering medication | |||||
| Belatacept | 4 (57%) | 3 (50%) | 4 (67%) | 4 (67%) | 3 (60%) |
| Switch | 4 (50%) | 4 (67%) | 5 (83%) | 4 (67%) | 4 (80%) |
| CNI | 2 (40%) | 3 (60%) | 2 (40%) | 2 (40%) | 1 (25%) |
| NODAT (n (%)) | |||||
| Belatacept | 0 | 0 | 0 | 0 | 0 |
| Switch | 1 (14%) | 1 (17%) | 1 | 1 | 1 (20%) |
| CNI | 0 | 0 | 0 | 0 | 0 |
| Number of patients | |||||
| Belatacept | 7 | 6 | 6 | 6 | 5 |
| Switch | 8 | 6 | 6 | 6 | 5 |
| CNI | 5 | 5 | 5 | 5 | 4 |
| Belatacept group | Deaths: 2 | - Sepsis due to pleural empyema after valve replacement (year 2) |
| - Bronchial cancer (year 8) | ||
| Graft loss: 0 | ||
| Switch group | Deaths: 2 | - Sudden heart failure (month 20) |
| - Bronchial cancer (year 3) | ||
| Graft loss: 1 | -Recurrence of IgA nephropathy (year 9) | |
| CNI group | Deaths: 1 | - Decompensation of aortic valve stenosis (year 9) |
| Graft loss: 0 |