Figure 1. Proportion of patients free of BV relapse after three-cycle and six-cycle periods of prophylaxis (Intention-to-treat population, n = 142).
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 5, Number 4, August 2013, pages 309-315
Efficacy of Vitamin C Vaginal Tablets as Prophylaxis for Recurrent Bacterial Vaginosis: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial
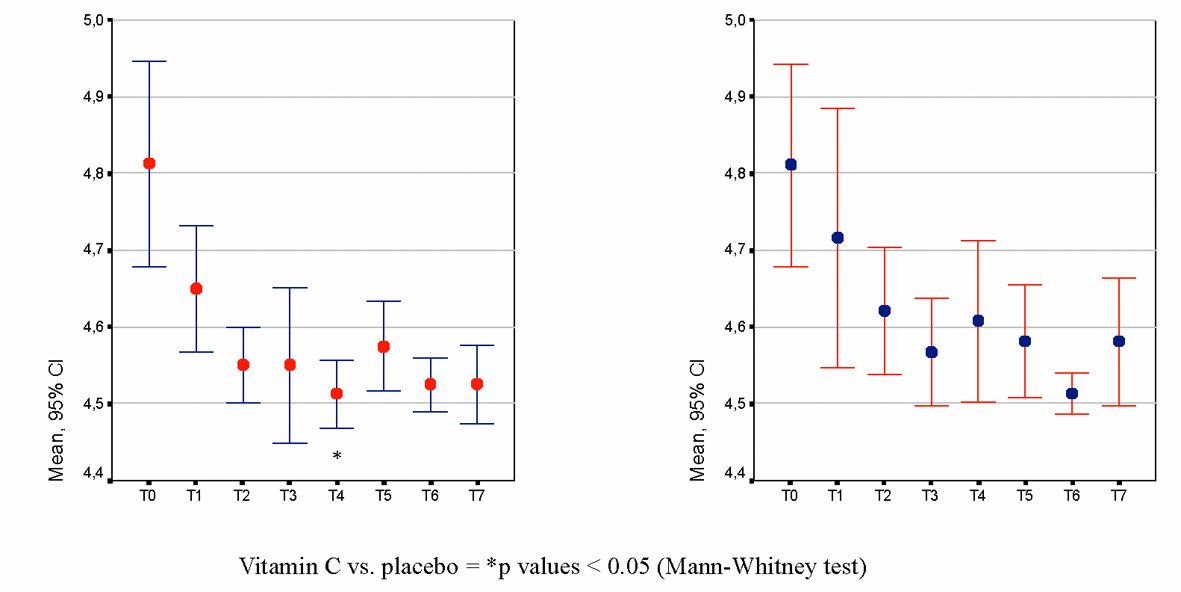
Figure

Tables
| Variable | Vitamin C (n = 74) | Placebo (n = 68) | Total (n = 142) |
|---|---|---|---|
| Demographic data | 74 | 68 | 142 |
| Age (years) (mean ± SD) | 31.2 ± 7.7 | 31.0 ± 7.2 | 31.1 ± 7.4 |
| Body weight (kg) (mean ± SD) | 61.0 ± 9.4 | 60.5 ± 8.4 | 60.8 ± 8.9 |
| Height (cm) (mean ± SD) | 165.3 ± 5.7 | 165.9 ± 6.2 | 165.6 ± 5.9 |
| Itching (mean ± SD) | 0.15 ± 0.4 | 0.16 ± 0.37 | 0.15 ± 0.38 |
| Burning (mean ± SD) | 0.14 ± 0.34 | 0.13 ± 0.38 | 0.13 ± 0.36 |
| Dysuria (mean ± SD) | 0.03 ± 0.16 | 0.04 ± 0.21 | 0.04 ± 0.19 |
| Odour (mean ± SD) | 0.09 ± 0.3 | 0.19 ± 0.43 | 0.14 ± 0.37 |
| Erythema (mean ± SD) | 0.01 ± 0.12 | 0.06 ± 0.24 | 0.04 ± 0.19 |
| Oedema (mean ± SD) | 0.00 ± 0.00 | 0.03 ± 0.17 | 0.01 ± 0.12 |
| Fissures (mean ± SD) | 0.00 ± 0.00 | 0.01 ± 0.12 | 0.01 ± 0.08 |
| Adverse event | Treatment group | |
|---|---|---|
| Vitamin C (n = 74) | Placebo (n = 68) | |
| Burning, itching, skin irritation | 3 (4.0%) | 4 (5.9%) |
| Candidiasis | 1 (1.4%) | 1 (1.5%) |
| Cystitis | - | 1 (1.5%) |
| Nausea | - | 1 (1.5%) |
| Bronchitis | 1 (1.4%) | - |
| Major depression | - | 1 (1.5%) |
| Flu | - | 1 (1.5%) |