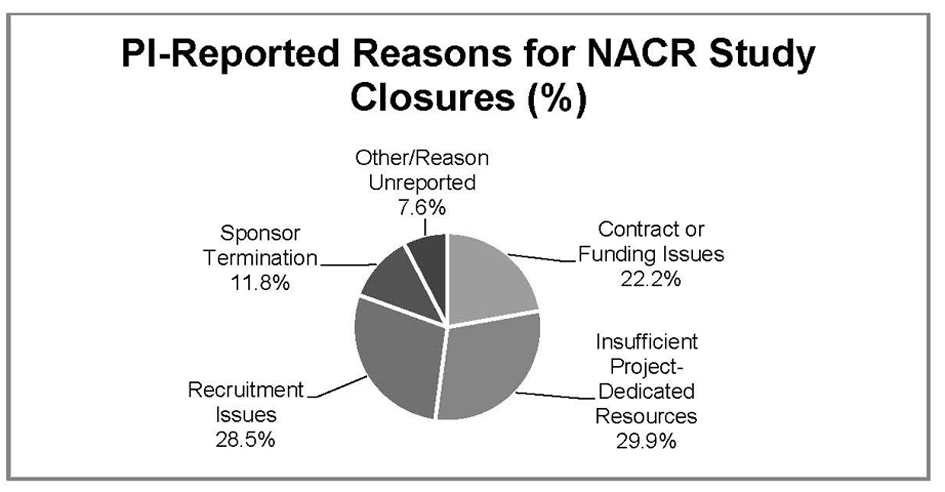
Figure 1. PI reported reasons for biomedical NACR study closures Northwestern University, 2010 - 2011.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 5, Number 3, June 2013, pages 185-193
Characteristics and Causes for Non-Accrued Clinical Research (NACR) at an Academic Medical Institution
Figure

Tables
| NACR | ACR | Total | |||||
|---|---|---|---|---|---|---|---|
| Sponsorship | NACR | Expedited Review | Full Panel Review | ACR | Expedited Review | Full Panel Review | NACR & ACR (% of Total) |
| Sponsored Studies | |||||||
| Industry-funded | 60 | 6 | 54 | 212 | 35 | 177 | 272 (32.0%) |
| Federally-funded | 30 | 9 | 21 | 140 | 63 | 77 | 170 (20.0%) |
| Foundation-funded | 13 | 8 | 5 | 55 | 36 | 19 | 68 (8.0%) |
| Total Sponsored Studies | 103 | 23 | 80 | 407 | 134 | 273 | 510 (60.0%) |
| Non-Sponsored Studies | |||||||
| Total Non-Sponsored Studies | 41 | 27 | 14 | 297 | 239 | 58 | 338 (40.0%) |
| Total | 144 | 50 | 94 | 704 | 373 | 331 | 848 (100.0%) |
| Reporting Entity | No NACR | ≤ 1 NACR | Only NACR | Total |
|---|---|---|---|---|
| Principal Investigator | 224 (68.3%) | 71 (21.7%) | 33 (10.0%) | 328 |
| Department/ Division | 31 (42.3%) | 34 (48.6) | 5 (7.1%) | 70 |
| Submission Type (# of deferred submissions) | |||||
|---|---|---|---|---|---|
| Sponsorship | New Project | Continuing Review | Revision | Safety-Other | Total |
| This includes studies that require written or verbal consent as well as projects that have waived consent. | |||||
| Industry-funded | 54 (10) | 19 (1) | 35 | 11 | 119 (11) |
| Federally-funded | 21 (4) | 4 | 5 | 2 | 32 (4) |
| Foundation-funded | 5 (1) | 0 | 0 | 0 | 5 (1) |
| Non-Sponsored Studies | 14 (8) | 1 | 3 (3) | 0 | 18 (11) |
| Total | 94 (23) | 24 (1) | 43 (3) | 13 | 174 (27) |
| Submission Type (# of deferred submissions) | ||||||
|---|---|---|---|---|---|---|
| Sponsorship | New Project | Continuing Review | Revision | Safety-Other | Termination | Total |
| This includes studies that require written or verbal consent as well as projects that have waived consent. | ||||||
| Industry-funded | 6 | 19 | 129 | 12 | 60 | 222 |
| Federally-funded | 9 | 34 | 57 | 0 | 30 | 131 |
| Foundation-funded | 8 | 8 | 10 | 0 | 13 | 39 |
| Non-Sponsored Studies | 27 | 55 | 58 | 1 | 41 | 182 |
| Total | 50 | 116 | 254 | 13 | 144 | 577 |
| Reason for NACR | Non-Sponsored | Industry-Funded | Federally-Funded | Foundation-Funded | Total (% of NACR) |
|---|---|---|---|---|---|
| Insufficient Study-Dedicated Resources (n = 43) | 28 | 4 | 8 | 3 | 43 (30.0%) |
| Recruitment Issues (n = 41) | 2 | 30 | 9 | 0 | 41 (28.4%) |
| Contract of Funding Issues (n = 32) | 6 | 11 | 9 | 6 | 32 (22.2%) |
| Sponsor Termination (n = -17) | 2 | 11 | 3 | 1 | 17 (11.8%) |
| Other/Reason Unreported (n = 11) | 3 | 4 | 1 | 3 | 11 (7.6%) |
| Total | 41 | 60 | 30 | 13 | 144 (100.0%) |