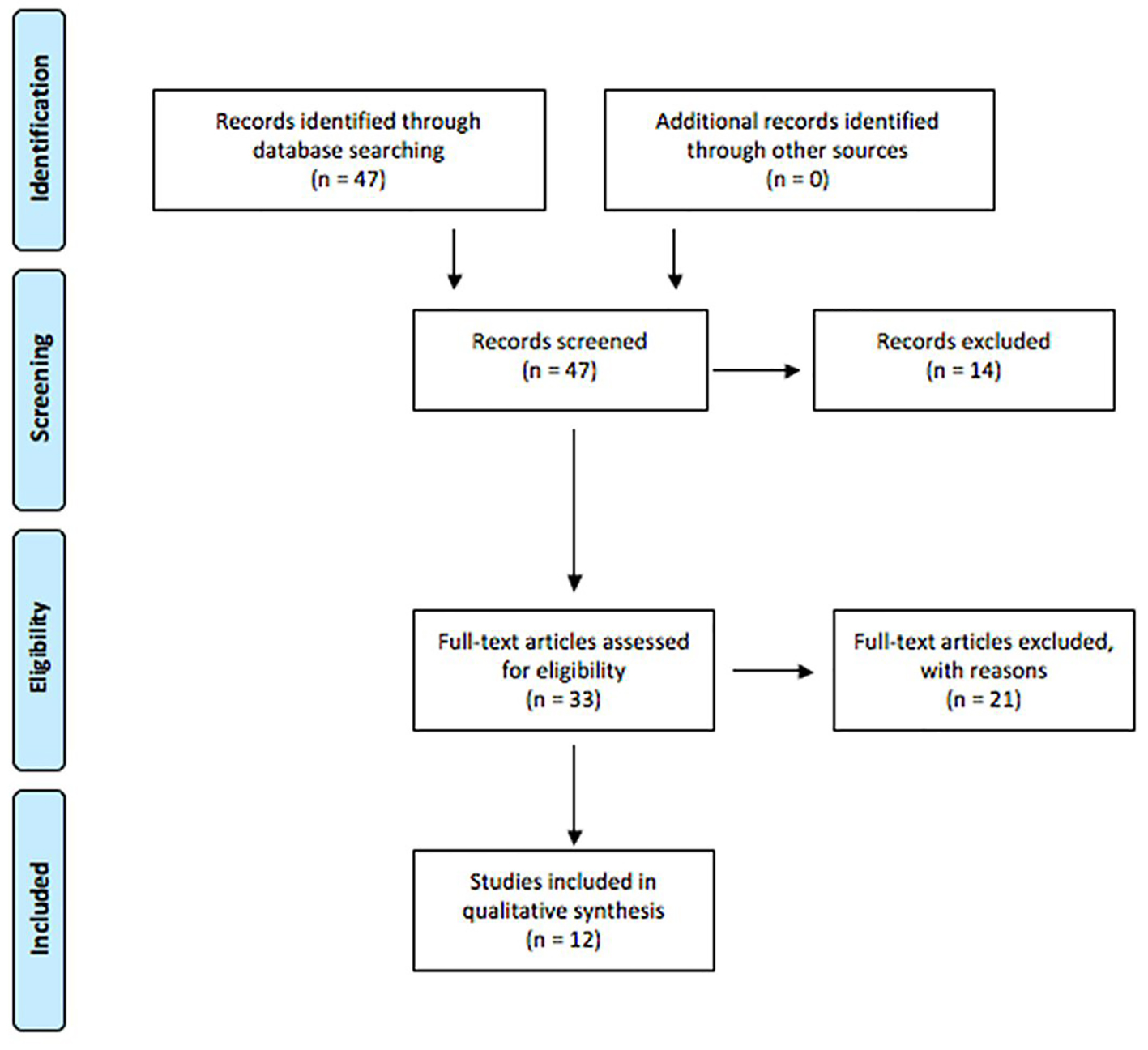
Figure 1. PRISMA diagram for article management.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 10, Number 3, March 2018, pages 159-165
A Systematic Review Regarding Tonometry and the Transmission of Infectious Diseases
Figure

Tables
| Author and year | Objective | Study design | Population size | Result | Quality score |
|---|---|---|---|---|---|
| The table demonstrates the results regarding the transmissibility of HIV, hepatitis B, hepatitis C, and prion diseases through human tears. | |||||
| Amin et al (2003) [13] | To determine the potential risk of horizontal transmission of proteinaceous material through the use of contact tonometry. How tested? | Experimental | 12 individuals | Tonometer tips can contribute to the transmission of proteinaceous material. Rinsing the tonometer tips in water reduced carryover of material. | 7/18 |
| Britt et al (1991) [16] | To determine whether human immunodeficiency virus (HIV) is dispersed through droplets during air puff tonometry. | Experimental | four individuals | One group received eye drops prior to the use of micro-aerosol tonometry, and one group did not. Scatter of eye droplets occurred in both groups during air puff tonometry. | 5/18 |
| Darrell and Jacob (1978) [17] | To determine the presence of hepatitis B in human tears and the risk of transferring this infection through contact lens fitting and contact tonometry. | Experimental | 33 individuals | Eighteen patients were tested with positive serum hepatitis B, and of these 18 individuals, 10 had hepatitis B in tears collected using the Schirmer strip or through the contact lens method. The 15 controls had no hepatitis B detected in their tears. | 5/18 |
| Feucht et al (1995) [18] | To determine whether the tear fluid of hepatitis C virus carriers is infectious. | Experimental | 76 individuals | All 76 patients chronically infected with hepatitis C were positive for hepatitis C RNA within tear fluid. | 6/18 |
| Komatsu et al (2012) [19] | To assess the possibility of transmission of hepatitis B virus in tears, urine, saliva and sweat. Additionally, the infectivity of tears from hepatitis B carriers was analyzed. | Experimental | 47 individuals | The transmissibility of this infectious disease from tears was studied in a chimeric mouse model. Hepatitis B tear specimens collected from a child and injected intravenously into two chimeric mice. One week following inoculation, both mice tested positive for hepatitis B DNA in their serum. | 11/18 |
| Moniz et al (1981) [20] | To determine whether tears contain hepatitis B surface antigen, to determine whether hepatitis B surface antigen can be detected on the tonometer tip following contact with carriers, and to determine whether washing the prism with running water is effective for removing hepatitis B surface antigen from contaminated tonometers. | Experimental | 31 individuals | Detection of hepatitis B surface antigen was only found in the conjunctival fluid of carriers who had higher titers of hepatitis B surface antigen in their sera. Rinsing the tonometer in water for ten seconds adequately removes detectable amounts of hepatitis B surface antigen from the tonometer. | 6/18 |
| Su et al (1994) [21] | To determine the transmissibility of hepatitis B DNA in human tears. | Experimental | 36 individuals | Detected that both patients with acute hepatitis B (two individuals) had tear specimens that were positive for HBV DNA, and 16 of the 34 carriers of chronic HBV had tear specimens that tested positive repeatedly for HBV DNA. The tear specimens of 10 of the 34 individuals with chronic HBV repeatedly tested negative, and the remaining eight tear specimens were equivocal. | 8/18 |
| Author and year | Objective | Study design | Population size | Sterilization techniques mentioned | Result | Quality score |
|---|---|---|---|---|---|---|
| The following table demonstrates the results regarding tonometer sterilization techniques against HIV, hepatitis B, hepatitis C, and prion disease. | ||||||
| Segal et al (2001) [22] | To compare decontamination methods for Goldmann tonometers containing hepatitis C virus. | Experimental | one individual | The sterilization techniques for the tonometer compared included: a 5 min soak in 3% hydrogen peroxide or 70% isopropyl alcohol (wipes or soak), dry gauze wipes, and a wash in cold water. | The isopropyl alcohol soak and cold water washed removed the greatest percentage of hepatitis C virus from the tonometer. A 5 min soak in either solution followed by washing in cold water is most effective at reducing the risk of hepatitis C virus transmission. | 7/18 |
| Pepose et al (1989) [9] | To test several protocols for the disinfection of Goldmann tonometer tips against HIV-1, herpes simplex virus type 1, and HSV type 2. | Experimental | one individual | The sterilization techniques for the tonometer compared included: hydrogen peroxide, isopropyl alcohol treatments, sterile gauze and sterile tissue. | The hydrogen peroxide and isopropyl alcohol treatments were both effective for disinfection of the tonometer tip against HIV. Wiping the tip with a sterile gauze or tissue was not effective. | 6/18 |
| Lim et al (2003) [8] | To evaluate the adequacy of current decontamination techniques for the Goldmann tonometer against variant Creutzfeldt Jakob disease (vCJD). | Experimental | 69 individuals | The sterilization technique mentioned involved: wiping the tonometer head with tissue, wiping with tissue + sodium hypochlorite solution for 10 min, under water with tissue, and then placing in sodium hypochlorite for 10 min. | Patients using eye drops desquamated an increased amount of corneal epithelial cells using Goldmann tonometry compared to patients who did not. Wiping or washing the tonometer head reduced but did not eliminate the number of cells significantly. | 11/18 |
| Su et al (1994) [23] | To determine which tonometer disinfection methods are most effective against hepatitis B. | Experimental | - | The disinfection procedures outlined in this study included: soap and water wash, a 70% isopropanol wipe, a 70% ethanol wipe, a wipe with distilled water, a 500 ppm chlorine soak (10 min), a 11% glutareldehyde soak (10 min), and a tap water rinse. | A soap and water wash removed all of the detectable hepatitis B DNA. A 500 ppm chlorine soak removed the detectable HBV DNA in some of the trials. The other methods resulted in residual HBV DNA remaining. | 4/18 |